The Structure Basis of Phytochemicals as Metabolic Signals for Combating Obesity
- PMID: 35769384
- PMCID: PMC9234462
- DOI: 10.3389/fnut.2022.913883
The Structure Basis of Phytochemicals as Metabolic Signals for Combating Obesity
Abstract
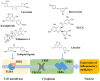
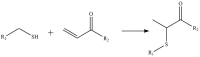
The consumption of phytochemicals, bioactive compounds in fruits and vegetables, has been demonstrated to ameliorate obesity and related metabolic symptoms by regulating specific metabolic pathways. This review summarizes the progress made in our understanding of the potential of phytochemicals as metabolic signals: we discuss herein selected molecular mechanisms which are involved in the occurrence of obesity that may be regulated by phytochemicals. The focus of our review highlights the regulation of transcription factors toll like receptor 4 (TLR4), nuclear factor (erythroid-derived 2)-like 2 (Nrf2), the peroxisome proliferator-activated receptors (PPARs), fat mass and obesity-associated protein (FTO) and regulation of microRNAs (miRNA). In this review, the effect of phytochemicals on signaling pathways involved in obesity were discussed on the basis of their chemical structure, suggesting molecular mechanisms for how phytochemicals may impact these signaling pathways. For example, compounds with an isothiocyanate group or an α, β-unsaturated carbonyl group may interact with the TLR4 signaling pathway. Regarding Nrf2, we examine compounds possessing an α, β-unsaturated carbonyl group which binds covalently with the cysteine thiols of Keap1. Additionally, phytochemical activation of PPARs, FTO and miRNAs were summarized. This information may be of value to better understand how specific phytochemicals interact with specific signaling pathways and help guide the development of new drugs to combat obesity and related metabolic diseases.
Keywords: metabolic signals; obesity; phytochemicals; structure; transcription factors.
Copyright © 2022 Li, Zheng, Zhang, Deng and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals.Planta Med. 2008 Oct;74(13):1526-39. doi: 10.1055/s-0028-1088302. Epub 2008 Oct 20. Planta Med. 2008. PMID: 18937164 Review.
-
Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals.Mol Nutr Food Res. 2016 Aug;60(8):1731-55. doi: 10.1002/mnfr.201501017. Epub 2016 Jun 1. Mol Nutr Food Res. 2016. PMID: 27523917 Review.
-
Discovery of Keap1-Nrf2 small-molecule inhibitors from phytochemicals based on molecular docking.Food Chem Toxicol. 2019 Nov;133:110758. doi: 10.1016/j.fct.2019.110758. Epub 2019 Aug 11. Food Chem Toxicol. 2019. PMID: 31412289 Free PMC article.
-
Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals.Toxicol Lett. 2014 Aug 17;229(1):73-84. doi: 10.1016/j.toxlet.2014.05.018. Epub 2014 May 27. Toxicol Lett. 2014. PMID: 24875534 Review.
-
Nutrigenomic Functions of PPARs in Obesogenic Environments.PPAR Res. 2016;2016:4794576. doi: 10.1155/2016/4794576. Epub 2016 Nov 30. PPAR Res. 2016. PMID: 28042289 Free PMC article. Review.
Cited by
-
Association of Plant-Based and High-Protein Diets with a Lower Obesity Risk Defined by Fat Mass in Middle-Aged and Elderly Persons with a High Genetic Risk of Obesity.Nutrients. 2023 Feb 20;15(4):1063. doi: 10.3390/nu15041063. Nutrients. 2023. PMID: 36839421 Free PMC article.
-
Microemulsion-based drug delivery system identifies pepper alkaloids as anti-obesity compounds.Acta Pharmacol Sin. 2025 Aug;46(8):2310-2322. doi: 10.1038/s41401-025-01521-x. Epub 2025 Mar 20. Acta Pharmacol Sin. 2025. PMID: 40113987
-
Immunomodulation by cannabidiol in bovine primary ruminal epithelial cells.BMC Vet Res. 2023 Oct 16;19(1):208. doi: 10.1186/s12917-023-03756-4. BMC Vet Res. 2023. PMID: 37845710 Free PMC article.
-
New Insights for Polyphenolic Compounds as Naturally Inspired Proteasome Inhibitors.Pharmaceuticals (Basel). 2023 Dec 11;16(12):1712. doi: 10.3390/ph16121712. Pharmaceuticals (Basel). 2023. PMID: 38139838 Free PMC article.
-
Ellagic Acid Modulates Necroptosis, Autophagy, Inflammations, and Stress to Ameliorate Nonalcoholic Liver Fatty Disease in a Rat Model.Food Sci Nutr. 2025 Jan 19;13(1):e4694. doi: 10.1002/fsn3.4694. eCollection 2025 Jan. Food Sci Nutr. 2025. PMID: 39830906 Free PMC article.
References
-
- Konopelniuk VV, Goloborodko II, Ishchuk TV, Synelnyk TB, Ostapchenko LI, Spivak MY, et al. . Efficacy of Fenugreek-based bionanocomposite on renal dysfunction and endogenous intoxication in high-calorie diet-induced obesity rat model-comparative study. EPMA J. (2017) 8:377–90. 10.1007/s13167-017-0098-2 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

