Impact of Food Matrices on Digestibility of Allergens and Poorly Allergenic Homologs
- PMID: 35769559
- PMCID: PMC9234860
- DOI: 10.3389/falgy.2022.909410
Impact of Food Matrices on Digestibility of Allergens and Poorly Allergenic Homologs
Abstract
Background: Protease resistance is considered a risk factor for allergenicity of proteins, although the correlation is low. It is nonetheless a part of the weight-of-evidence approach, proposed by Codex, for assessing the allergenicity risk of novel food proteins. Susceptibility of proteins to pepsin is commonly tested with purified protein in solution.
Objective: Food proteins are rarely consumed in purified form. Our aim was to evaluate the impact of experimental and endogenous food matrices on protease susceptibility of homologous protein pairs with different degrees of allergenicity.
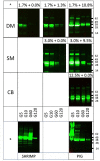
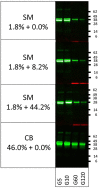
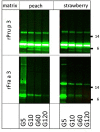
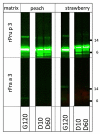
Methods: Porcine and shrimp tropomyosin (ST) were subjected to sequential exposure to amylase, pepsin, and pancreatin in their respective endogenous matrix (pork tenderloin/boiled shrimp) and in three different experimental matrices (dessert mousse [DM], soy milk [SM], and chocolate bar [CB]). Digestion was monitored by immunoblotting using tropomyosin-specific antibodies. Recombinant peach and strawberry lipid transfer protein were biotinylated, spiked into both peach and strawberry fruit pulp, and subjected to the same sequential digestion protocol. Digestion was monitored by immunoblotting using streptavidin for detection.
Results: Chocolate bar, and to a lesser extent SM, had a clear protective effect against pepsin digestion of porcine tropomyosin (PT) and to a lesser extent of ST. Increased resistance was associated with increased protein content. Spiking experiments with bovine serum albumin (BSA) confirmed the protective effect of a protein-rich matrix. The two tropomyosins were both highly resistant to pepsin in their protein-rich and lean native food matrix. Pancreatin digestion remained rapid and complete, independent of the matrix. The fat-rich environment did not transfer protection against pepsin digestion. Spiking of recombinant peach and strawberry lipid transfer proteins into peach and strawberry pulp did not reveal any differential protective effect that could explain differences in allergenicity of both fruits.
Conclusions: Protein-rich food matrices delay pepsin digestion by saturating the protease. This effect is most apparent for proteins that are highly pepsin susceptible in solution. The inclusion of food matrices does not help in understanding why some proteins are strong primary sensitizers while homologs are very poor allergens. Although for induction of symptoms in food allergic patients (elicitation), a protein-rich food matrix that may contribute to increased risk, our results indicate that the inclusion of food matrices in the weight-of-evidence approach for estimating the potential risks of novel proteins to become allergens (sensitization), is most likely of very limited value.
Keywords: allergen exposure; allergenicity; food matrix; protease resistance; risk assessment.
Copyright © 2022 Akkerdaas, Cianferoni, Islamovic, Kough, Ladics, McClain, Poulsen, Silvanovich, Pereira Mouriès and van Ree.
Conflict of interest statement
EI was employed by BASF Corporation. GL was employed by Dupont Nutrition and Biosciences. SM was employed by SAS Institute. AS was employed by Bayer U.S. Crop Science. RR was reported consultancy for HAL Allergy, Citeq, Angany, Reacta Healthcare, Mission MightyMe, AB Enzymes. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Codex Alimentarius C. Guideline for the conduct of food safety assessment of foods derived from recombinant-DNA plants. CAC/GL. (2003) 45:1–13.
LinkOut - more resources
Full Text Sources

