Immune Activation, Exhaustion and Senescence Profiles as Possible Predictors of Cancer in Liver Transplanted Patients
- PMID: 35769714
- PMCID: PMC9235349
- DOI: 10.3389/fonc.2022.899170
Immune Activation, Exhaustion and Senescence Profiles as Possible Predictors of Cancer in Liver Transplanted Patients
Abstract
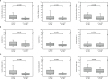
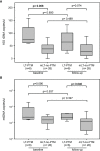
Liver transplanted (LT) patients for hepatocellular carcinoma (LT-HCC) or for other causes (LT-no-HCC) may develop post-transplantation malignancies. Although immune activation and senescence are frequently implicated in cancer development, no data is available on their possible role as biomarkers predictive of tumor onset in this setting. A total of 116 patients were investigated: the 45 LT-HCC patients were older than the 71 LT-non-HCC (p=0.011), but comparable for sex, HCV, HBV infection and immunosuppressive treatment. At baseline, the numbers of activated and senescent-like circulating cells were significantly higher in LT-HCC patients than in LT-no-HCC ones. After a median follow-up of 26.8 months, 6 post-transplant malignancies (PTM) occurred: 4 in LT-HCC (8.9%) and 2 in LT-no-HCC (2.8%) patients. Overall, subjects with high percentages of activated and exhausted T and B cells at baseline were at higher risk of PTM. Notably, within the LT-HCC group, a higher percentage of senescence-like T cells was also associated with cancer development. Moreover, patients with PTM had higher telomere erosion and higher levels of circulating PAMPs (16S rDNA) and DAMPs (mtDNA) when compared with matched patients without PTM. Overall, these findings suggest that immune activation and exhaustion may be useful to predict the risk of PTM occurrence, regardless of the cause of transplantation. In LT-HCC, T-cell senescence represents an additional risk factor for tumor onset.
Keywords: biological predictors; hepatocellular carcinoma; immune activation; immune senescence; post-transplant malignancy.
Copyright © 2022 Petrara, Shalaby, Ruffoni, Taborelli, Carmona, Giunco, Del Bianco, Piselli, Serraino, Cillo, Dolcetti, Burra and De Rossi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Hepatocellular Carcinoma in the Setting of Chronic Hepatitis B Virus Infection: Tumor Recurrence and Survival Rates After Liver Transplantation.Transplant Proc. 2015 Jul-Aug;47(6):1939-44. doi: 10.1016/j.transproceed.2015.02.021. Transplant Proc. 2015. PMID: 26293077
-
Liver transplantation for hepatitis B virus: Decreasing indication and changing trends.World J Gastroenterol. 2015 Jul 14;21(26):8140-7. doi: 10.3748/wjg.v21.i26.8140. World J Gastroenterol. 2015. PMID: 26185387 Free PMC article.
-
Assessing Competing Risks for Death Following Liver Transplantation for Hepatocellular Carcinoma.Dig Dis Sci. 2019 Apr;64(4):1001-1007. doi: 10.1007/s10620-019-05538-1. Dig Dis Sci. 2019. PMID: 30852770 Review.
-
Clinical efficacy of direct-acting antiviral therapy for recurrent hepatitis C virus infection after liver transplantation in patients with hepatocellular carcinoma.World J Hepatol. 2020 Sep 27;12(9):628-640. doi: 10.4254/wjh.v12.i9.628. World J Hepatol. 2020. PMID: 33033569 Free PMC article.
-
Prophylactic liver transplantation for high-risk recurrent hepatocellular carcinoma.World J Hepatol. 2016 Nov 8;8(31):1309-1317. doi: 10.4254/wjh.v8.i31.1309. World J Hepatol. 2016. PMID: 27872682 Free PMC article. Review.
Cited by
-
Ferroptosis in liver cancer: a key role of post-translational modifications.Front Immunol. 2024 Apr 8;15:1375589. doi: 10.3389/fimmu.2024.1375589. eCollection 2024. Front Immunol. 2024. PMID: 38650929 Free PMC article. Review.
-
Cellular Senescence in Liver Cancer: How Dying Cells Become "Zombie" Enemies.Biomedicines. 2023 Dec 21;12(1):26. doi: 10.3390/biomedicines12010026. Biomedicines. 2023. PMID: 38275386 Free PMC article. Review.
-
Global, regional and country burden of high BMI-related liver cancer among individuals aged above 70: trends from 1990 to 2021 and projections to 2044.Front Public Health. 2025 Mar 20;13:1523578. doi: 10.3389/fpubh.2025.1523578. eCollection 2025. Front Public Health. 2025. PMID: 40182530 Free PMC article.
References
-
- Centro Nazionale Trapianti. Ministero della Salute. Available at: http://www.trapianti.salute.gov.it.
LinkOut - more resources
Full Text Sources

