Increased Expression of Mitochondrial UQCRC1 in Pancreatic Cancer Impairs Antitumor Immunity of Natural Killer Cells via Elevating Extracellular ATP
- PMID: 35769718
- PMCID: PMC9234308
- DOI: 10.3389/fonc.2022.872017
Increased Expression of Mitochondrial UQCRC1 in Pancreatic Cancer Impairs Antitumor Immunity of Natural Killer Cells via Elevating Extracellular ATP
Abstract
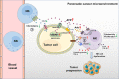
Pancreatic cancer (PC) is one of the most lethal malignancies characterized by a highly immunosuppressive tumor microenvironment (TME). Previously, we have reported that ubiquinol-cytochrome c reductase core protein I (UQCRC1), a key component of mitochondrial complex III, is generally upregulated in PC and produces extracellular ATP (eATP) to promote PC progression. Here, we sought to investigate whether the oncogenic property of UQCRC1 is generated through its effects on natural killer (NK) cells in the TME. We found that UQCRC1 overexpression in PC cells inhibited cytotoxicity of NK cells, as well as the infiltration of NK cells toward PC, whereas knockdown of UQCRC1 enhanced the cytotoxicity and chemotaxis of NK cells. Adoptive NK cell therapy in the subcutaneous mouse model and CIBERSORTx analysis with human PC specimens confirmed UQCRC1 elicited immunosuppressive effects on NK cells. Such UQCRC1-induced impairment of NK cells was mediated by eATP and its metabolite adenosine via P2Y11R and A2AR, respectively. Mechanistically, we found the UQCRC1/eATP axis reduced the expression of chemokine CCL5 in cancer cells and altered the balance of activating receptor DNAM-1 and inhibitory receptor CD96 on NK-92MI cells, resulting in decreased chemotaxis and exhausted phenotype of NK-92MI cells. Taken together, our study provides the evidence to support a novel mechanism by which energy metabolism change in cancer cells remodels the TME and impedes NK cell surveillance. It also suggests that targeting UQCRC1 may be a potential combined strategy for PC immunotherapy.
Keywords: Extracellular adenosine triphosphate; NK cells; UQCRC1; extracellular adenosine; pancreatic cancer.
Copyright © 2022 Cong, Gao, Wang, Du, Li, Li, Li, Liang, Zhao, Yang, Gan and Tu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Mitochondrial Protein UQCRC1 is Oncogenic and a Potential Therapeutic Target for Pancreatic Cancer.Theranostics. 2020 Jan 12;10(5):2141-2157. doi: 10.7150/thno.38704. eCollection 2020. Theranostics. 2020. PMID: 32089737 Free PMC article.
-
A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment.Cancer Res. 2018 Feb 15;78(4):1003-1016. doi: 10.1158/0008-5472.CAN-17-2826. Epub 2017 Dec 11. Cancer Res. 2018. PMID: 29229601
-
Biomimetic Nanocarriers Guide Extracellular ATP Homeostasis to Remodel Energy Metabolism for Activating Innate and Adaptive Immunity System.Adv Sci (Weinh). 2022 Jun;9(17):e2105376. doi: 10.1002/advs.202105376. Epub 2022 Apr 9. Adv Sci (Weinh). 2022. PMID: 35396800 Free PMC article.
-
DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy.Cancers (Basel). 2019 Jun 23;11(6):877. doi: 10.3390/cancers11060877. Cancers (Basel). 2019. PMID: 31234588 Free PMC article. Review.
-
Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects.Cancers (Basel). 2019 Apr 1;11(4):461. doi: 10.3390/cancers11040461. Cancers (Basel). 2019. PMID: 30939820 Free PMC article. Review.
Cited by
-
Unveiling the immunosuppressive landscape of pancreatic ductal adenocarcinoma: implications for innovative immunotherapy strategies.Front Oncol. 2024 Mar 25;14:1349308. doi: 10.3389/fonc.2024.1349308. eCollection 2024. Front Oncol. 2024. PMID: 38590651 Free PMC article. Review.
-
RBM47 promotes cell proliferation and immune evasion by upregulating PDIA6: a novel mechanism of pancreatic cancer progression.J Transl Med. 2024 Dec 31;22(1):1164. doi: 10.1186/s12967-024-05970-6. J Transl Med. 2024. PMID: 39741300 Free PMC article.
-
Metabolic plasticity in pancreatic cancer: The mitochondrial connection.Mol Metab. 2025 Feb;92:102089. doi: 10.1016/j.molmet.2024.102089. Epub 2024 Dec 28. Mol Metab. 2025. PMID: 39736443 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

