PD-1 blockade synergizes with oxaliplatin-based, but not cisplatin-based, chemotherapy of gastric cancer
- PMID: 35769948
- PMCID: PMC9235886
- DOI: 10.1080/2162402X.2022.2093518
PD-1 blockade synergizes with oxaliplatin-based, but not cisplatin-based, chemotherapy of gastric cancer
Abstract
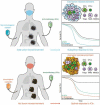
Preclinical experimentation revealed that established cancers treated with the immunogenic cell death (ICD) inducer oxaliplatin are sensitized to immune checkpoint inhibitors targeting PD-1. In contrast, no such sensitizing effect is observed when cisplatin, a non-immunogenic cell death inducer is used. Two randomized phase III clinical trials targeting unresectable gastric and gastro-esophageal junction carcinomas apparently validate this observation. Thus, oxaliplatin-based chemotherapy (together with capecitabine or 5-fluorouracil plus leucovorin) favorably interacted with nivolumab, yielding improved outcome. In contrast, the outcome of cisplatin-based chemotherapy (together with capecitabine or 5-fluorouracil) failed to be improved by concomitant treatment with pembrolizumab. These clinical findings underscore the importance of choosing appropriate ICD-inducing cytotoxicants for the development of chemoimmunotherapeutic regimens. Unfortunately, the FDA and EMA have approved PD-1 blockade in combination with "platinum-based chemotherapy" without specifying the precise nature of the platinum-containing drug. This is a non sequitur. Based on the available clinical data, such approvals should be restricted to the use of oxaliplatin.
Keywords: Clinical trial; Immunotherapy.
© 2022 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
GK and OK are cofounders of Samsara Therapeutics. GK is a cofounder of Therafast Bio. AH participates on data safety monitoring or consulting and advisory boards for Amgen, BMS, Basilea, Incyte, Servier, QED Therapeutics, Tahio, and Relay Therapeutics.
Figures
References
-
- Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141–148. doi:10.1093/annonc/mdu472. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
