Brain-Inspired Spiking Neural Network Controller for a Neurorobotic Whisker System
- PMID: 35770277
- PMCID: PMC9234954
- DOI: 10.3389/fnbot.2022.817948
Brain-Inspired Spiking Neural Network Controller for a Neurorobotic Whisker System
Abstract
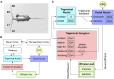
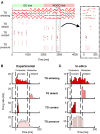
It is common for animals to use self-generated movements to actively sense the surrounding environment. For instance, rodents rhythmically move their whiskers to explore the space close to their body. The mouse whisker system has become a standard model for studying active sensing and sensorimotor integration through feedback loops. In this work, we developed a bioinspired spiking neural network model of the sensorimotor peripheral whisker system, modeling trigeminal ganglion, trigeminal nuclei, facial nuclei, and central pattern generator neuronal populations. This network was embedded in a virtual mouse robot, exploiting the Human Brain Project's Neurorobotics Platform, a simulation platform offering a virtual environment to develop and test robots driven by brain-inspired controllers. Eventually, the peripheral whisker system was adequately connected to an adaptive cerebellar network controller. The whole system was able to drive active whisking with learning capability, matching neural correlates of behavior experimentally recorded in mice.
Keywords: active whisking; central pattern generator (CPG); facial nuclei; neurorobotic architecture; point neuron model; trigeminal ganglion; trigeminal nuclei; vibrissae.
Copyright © 2022 Antonietti, Geminiani, Negri, D'Angelo, Casellato and Pedrocchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aguero C., Koenig N., Chen I., Boyer H., Peters S., Hsu J., et al. . (2015). Inside the virtual robotics challenge: simulating real-time robotic disaster response. IEEE Trans. Autom. Sci. Eng. 12, 494–506. 10.1109/TASE.2014.2368997 - DOI
-
- Ahissar E., Knutsen P. M. (2016). Vibrissal Location Coding. Paris: Atlantis Press. 725–735. 10.2991/978-94-6239-133-8_53 - DOI
LinkOut - more resources
Full Text Sources

