Amide Spectral Fingerprints are Hydrogen Bonding-Mediated
- PMID: 35770492
- PMCID: PMC9272440
- DOI: 10.1021/acs.jpclett.2c01277
Amide Spectral Fingerprints are Hydrogen Bonding-Mediated
Abstract
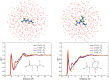
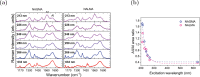
The origin of the peculiar amide spectral features of proteins in aqueous solution is investigated, by exploiting a combined theoretical and experimental approach to study UV Resonance Raman (RR) spectra of peptide molecular models, namely N-acetylglycine-N-methylamide (NAGMA) and N-acetylalanine-N-methylamide (NALMA). UVRR spectra are recorded by tuning Synchrotron Radiation at several excitation wavelengths and modeled by using a recently developed multiscale protocol based on a polarizable QM/MM approach. Thanks to the unparalleled agreement between theory and experiment, we demonstrate that specific hydrogen bond interactions, which dominate hydration dynamics around these solutes, play a crucial role in the selective enhancement of amide signals. These results further argue the capability of vibrational spectroscopy methods as valuable tools for refined structural analysis of peptides and proteins in aqueous solution.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

