Portable electronic bronchoscopy for clinical application: a multi-institutional randomized instrument validation study
- PMID: 35770525
- PMCID: PMC9252000
- DOI: 10.1177/03000605221108102
Portable electronic bronchoscopy for clinical application: a multi-institutional randomized instrument validation study
Abstract
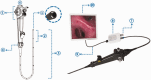
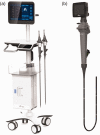
Objective: Electronic bronchoscopy is routinely used for the diagnosis and treatment of lung and bronchial disorders. However, the devices used are normally large and costly. Here, we evaluated the clinical effectiveness of a portable electronic bronchoscope produced by Zhejiang UE Medical Corp., the UE-EB.
Methods: We conducted a multi-institutional, randomized, single-blind, non-inferiority and parallel-group controlled clinical trial. Participants were randomly assigned 1:1 to the experimental group or control group. The primary indicator was the effectiveness of the device. Safety indicators were assessed from enrollment to 3 days after the operation.
Results: The UE-EB had good consistency between groups during the procedure, and the effective rate was 100.00% in both groups. The difference value (95% confidence interval) between the two groups was 0.00% (-5.45%, 5.45%), and the lower limit was greater than -10% (negative non-inferiority margin). There was also no difference between the two groups in terms safety indicators.
Conclusions: The portable electronic bronchoscope described in this study showed reliable effectiveness and safety. This device is worth promoting and applying in clinical practice.Research registry number: ZXLB20200295 (Zhejiang Medical Products Administration, China).
Keywords: Electronic bronchoscopy; clinical trial; effectiveness; instrument validation; multi-institution study; portable device; safety.
Conflict of interest statement
Figures
Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Wearable Integrated Volitional Control Electrical Stimulation Device as Treatment for Paresis of the Upper Extremity in Early Subacute Stroke Patients: A Randomized Controlled Non-inferiority Trial.Arch Phys Med Rehabil. 2024 Feb;105(2):227-234. doi: 10.1016/j.apmr.2023.08.031. Epub 2023 Sep 14. Arch Phys Med Rehabil. 2024. PMID: 37714508 Clinical Trial.
-
Efficacy and Safety of HSK3486 for Anesthesia/Sedation in Patients Undergoing Fiberoptic Bronchoscopy: A Multicenter, Double-Blind, Propofol-Controlled, Randomized, Phase 3 Study.CNS Drugs. 2022 Mar;36(3):301-313. doi: 10.1007/s40263-021-00890-1. Epub 2022 Feb 14. CNS Drugs. 2022. PMID: 35157236 Free PMC article. Clinical Trial.
-
Meta-analysis and systematic review of electronic bronchoscopy in refractory pneumonia.Ann Palliat Med. 2021 Sep;10(9):9889-9901. doi: 10.21037/apm-21-2133. Ann Palliat Med. 2021. PMID: 34628915
References
-
- Becker HD. Bronchoscopy: the past, the present, and the future. Clin Chest Med 2010; 31: 1–18. - PubMed
-
- Miller RJ, Casal RF, Lazarus DR, et al. Flexible Bronchoscopy. Clin Chest Med 2018; 39: 1–16. - PubMed
-
- Du Rand IA, Blaikley J, Booton R, et al. , for the British Thoracic Society Bronchoscopy Guideline Group. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013; 1–i44. - PubMed
-
- Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional Bronchoscopy. Am J Respir Crit Care Med 2020; 202: 29–50. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

