The nervous system during COVID-19: Caught in the crossfire
- PMID: 35770683
- PMCID: PMC9350403
- DOI: 10.1111/imr.13114
The nervous system during COVID-19: Caught in the crossfire
Abstract
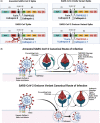
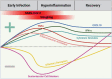
SARS-CoV-2, the virus that causes coronavirus disease (COVID)-19, has become a persistent global health threat. Individuals who are symptomatic for COVID-19 frequently exhibit respiratory illness, which is often accompanied by neurological symptoms of anosmia and fatigue. Mounting clinical data also indicate that many COVID-19 patients display long-term neurological disorders postinfection such as cognitive decline, which emphasizes the need to further elucidate the effects of COVID-19 on the central nervous system. In this review article, we summarize an emerging body of literature describing the impact of SARS-CoV-2 infection on central nervous system (CNS) health and highlight important areas of future investigation.
Keywords: COVID-19; NeuroCOVID; SARS-CoV-2; anosmia; neuroimmunology; neuroinflammation; olfactory system.
© 2022 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID‐19. Science. 2022;375(6585):1122‐1127. - PubMed
-
- Whitaker M, Elliott J, Bodinier B, et al. Variant‐specific symptoms of COVID‐19 among 1,542,510 people in England. Published online May 23, 2022:2022.05.21.22275368. 10.1101/2022.05.21.22275368 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

