Prevalence of antibodies against SARS-CoV-2 in the Norwegian population, August 2021
- PMID: 35770841
- PMCID: PMC9349429
- DOI: 10.1111/irv.13024
Prevalence of antibodies against SARS-CoV-2 in the Norwegian population, August 2021
Abstract
Background: One year into the COVID-19 pandemic, the cumulative number of confirmed COVID-19 cases in Norway was still low. In January 2021, when the Norwegian COVID-19 vaccination campaign started, the national seroprevalence estimate of SARS-CoV-2 antibodies was 3.2%. We have conducted a nationwide cross-sectional study in August 2021 to investigate the overall prevalence of SARS-CoV-2 antibodies in Norway after 8 months of COVID-19 mass vaccination and a third wave of SARS-CoV-2 infection.
Methods: Residual sera were collected from laboratories across Norway in August 2021. In IgG antibodies against the spike protein, the spike receptor binding domain (RBD) and the nucleocapsid protein of SARS-CoV-2 were measured by a bead-based flow cytometric assay.
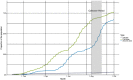
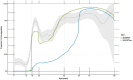
Results: In total, 1926 residual sera were collected from individuals aged 0-98 years; 55.1% were from women. The overall national estimated seroprevalence from vaccination and/or infection was 62.6% (credible interval [CrI] 60.1%-65.2%) based on having antibodies against both spike and RBD. Estimated seroprevalence increased with age. Among all samples, 11.7% had antibodies against nucleocapsid. For unvaccinated children <12 years, the seroprevalence estimate due to SARS-CoV-2 infection was 12.5% (95% CrI 9.3%-16.1%). Of seropositive samples from the unvaccinated children, 31.9% lacked anti-nucleocapsid antibodies.
Conclusions: The high overall SARS-CoV-2 seroprevalence estimates are in line with Norwegian registry data. Vaccination, not infection, contributed the most to the high seroprevalence in August 2021. Lack of antibodies against nucleocapsid should not automatically be interpreted as absence of previous infection as this could lead to underestimation of COVID-19 cases in seroprevalence studies.
Keywords: COVID-19; SARS-CoV-2; infection; nucleocapsid; seroprevalence; vaccination.
© 2022 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures
References
-
- Folkehelseinsituttet. Ukerapport—uke 29. www.fhi.no: Folkehelseinstituttet;2021.
-
- The Norwegian Government . Timeline: news from Norwegian Ministries about the coronavirus disease Covid‐19. https://www.regjeringen.no/en/topics/koronavirus-covid-19/timeline-for-n.... Accessed 25.03.22, 2022.
-
- Folkehelseinstituttet. Ukerapport—uke 31. www.fhi.no: Folkehelseinsituttet; 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

