Tumor Volume Reduction Rate to Induction Chemotherapy is a Prognostic Factor for Locally Advanced Head and Neck Squamous Cell Carcinoma: A Retrospective Cohort Study
- PMID: 35770906
- PMCID: PMC9252009
- DOI: 10.1177/15330338221107714
Tumor Volume Reduction Rate to Induction Chemotherapy is a Prognostic Factor for Locally Advanced Head and Neck Squamous Cell Carcinoma: A Retrospective Cohort Study
Abstract
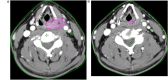
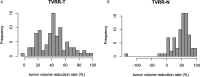
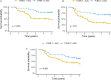
Introduction: Aim of this retrospective cohort study is to evaluate the prognostic value of tumor volume reduction rate status post-induction chemotherapy in locally advanced head and neck squamous cell carcinoma. Methods: Patients newly diagnosed from year 2007 to 2016 at a single center were included in this retrospective study. All patients had received induction Taxotere, Platinum, Fluorouracil followed by daily definitive intensity-modulated radiotherapy for 70 Gy in 35 fractions concurrent with or without cisplatin-based chemotherapy. Tumor volume reduction rate was measured and calculated by contrast-enhanced computed tomography images at diagnosis, and after at least 1 cycle of induction chemotherapy, and analyzed though a univariate and multivariate Cox regression model. Results: Ninety patients of the primary cancer sites at hypopharynx (31/90, 34.4%), oropharynx (29/90, 32.2%), oral cavity (19/90, 21.1%), and larynx (11/90, 12.2%) were included in this study, with a median follow-up time interval of 3.9 years. In multivariate Cox regression analysis, the tumor volume reduction rate of the primary tumor (TVRR-T) was also an independently significant prognostic factor for disease-free survival (DFS) (hazard ratio 0.77, 95% confidence interval 0.62-0.97; P-value = .02). Other factors including patient's age at diagnosis, the primary cancer site, and RECIST (Response Evaluation Criteria in Solid Tumors), were not significantly related. At a cutoff value using 50% in Kaplan-Meier survival analysis, the DFS was higher with TVRR-T ≥ 50% group (log-rank test, P = .024), and a trend of improved overall survival. (log-rank test, P = .069). Conclusion: TVRR-T is a probable prognostic factor for DFS. With a cut-off point of 50%, TVRR-T may indicate better DFS.
Keywords: early response; head and neck cancer; induction chemotherapy; locally advanced; squamous cell carcinoma; tumor volume; volume reduction.
Conflict of interest statement
Figures
References
-
- Ghi MG, Paccagnella A, Ferrari D, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(9):2206‐2212. - PubMed
-
- Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257‐264. - PubMed
-
- Hitt R, Grau JJ, López-Pousa A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25(1):216‐225. - PubMed
-
- Posner MR, Norris CM, Wirth LJ, et al. Sequential therapy for the locally advanced larynx and hypopharynx cancer subgroup in TAX 324: survival, surgery, and organ preservation. Ann Oncol. 2009;20(5):921‐927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

