Optical Modulation of Antibiotic Resistance by Photoswitchable Cystobactamids
- PMID: 35771231
- PMCID: PMC9804939
- DOI: 10.1002/chem.202201297
Optical Modulation of Antibiotic Resistance by Photoswitchable Cystobactamids
Abstract
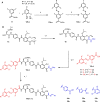
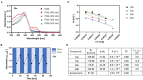
The rise of antibiotic resistance causes a serious health care problem, and its counterfeit demands novel, innovative concepts. The combination of photopharmacology, enabling a light-controlled reversible modulation of drug activity, with antibiotic drug design has led to first photoswitchable antibiotic compounds derived from established scaffolds. In this study, we converted cystobactamids, gyrase-inhibiting natural products with an oligoaryl scaffold and highly potent antibacterial activities, into photoswitchable agents by inserting azobenzene in the N-terminal part and/or an acylhydrazone moiety near the C-terminus, yielding twenty analogs that contain mono- as well as double-switches. Antibiotic and gyrase inhibition properties could be modulated 3.4-fold and 5-fold by light, respectively. Notably, the sensitivity of photoswitchable cystobactamids towards two known resistance factors, the peptidase AlbD and the scavenger protein AlbA, was light-dependent. While irradiation of an analog with an N-terminal azobenzene with 365 nm light led to less degradation by AlbD, the AlbA-mediated inactivation was induced. This provides a proof-of-principle that resistance towards photoswitchable antibiotics can be optically controlled.
Keywords: antibiotics; antimicrobial resistance; natural products; oligoarylamides; photopharmacology.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
GT, MB, and AR are coinventors on a patent application on synthetic cystobactamids.
Figures






Similar articles
-
Synthetic studies of cystobactamids as antibiotics and bacterial imaging carriers lead to compounds with high in vivo efficacy.Chem Sci. 2019 Dec 10;11(5):1316-1334. doi: 10.1039/c9sc04769g. Chem Sci. 2019. PMID: 34123255 Free PMC article.
-
Short Photoswitchable Antibacterial Peptides.ChemMedChem. 2020 Aug 19;15(16):1505-1508. doi: 10.1002/cmdc.202000280. Epub 2020 Jul 7. ChemMedChem. 2020. PMID: 32558320
-
Optical Control of Proteasomal Protein Degradation with a Photoswitchable Lipopeptide.Angew Chem Int Ed Engl. 2024 Feb 19;63(8):e202314791. doi: 10.1002/anie.202314791. Epub 2024 Jan 16. Angew Chem Int Ed Engl. 2024. PMID: 38109686 Free PMC article.
-
Strategies for the Discovery and Development of New Antibiotics from Natural Products: Three Case Studies.Curr Top Microbiol Immunol. 2016;398:339-363. doi: 10.1007/82_2016_498. Curr Top Microbiol Immunol. 2016. PMID: 27738913 Review.
-
Recent Progress in Regulating the Activity of Enzymes with Photoswitchable Inhibitors.Molecules. 2024 Sep 24;29(19):4523. doi: 10.3390/molecules29194523. Molecules. 2024. PMID: 39407453 Free PMC article. Review.
Cited by
-
Light-Based Anti-Biofilm and Antibacterial Strategies.Pharmaceutics. 2023 Aug 9;15(8):2106. doi: 10.3390/pharmaceutics15082106. Pharmaceutics. 2023. PMID: 37631320 Free PMC article. Review.
-
Transcription activation by the resistance protein AlbA as a tool to evaluate derivatives of the antibiotic albicidin.Chem Sci. 2023 Apr 17;14(19):5069-5078. doi: 10.1039/d3sc00955f. eCollection 2023 May 17. Chem Sci. 2023. PMID: 37206387 Free PMC article.
-
Synthesis of Tetra-ortho-Methoxylated Azobenzene Photoswitches via Sequential Catalytic C-H Activation and Methoxylation.J Org Chem. 2024 Dec 6;89(23):17141-17146. doi: 10.1021/acs.joc.4c01554. Epub 2024 Nov 8. J Org Chem. 2024. PMID: 39513681 Free PMC article.
-
Exploring antibiotic resistance with chemical tools.Chem Commun (Camb). 2023 May 18;59(41):6148-6158. doi: 10.1039/d3cc00759f. Chem Commun (Camb). 2023. PMID: 37039397 Free PMC article. Review.
References
-
- None
-
- Broichhagen J., Frank J. A., Trauner D., Acc. Chem. Res. 2015, 48, 1947–1960; - PubMed
-
- Lerch M. M., Hansen M. J., van Dam G. M., Szymanski W., Feringa B. L., Angew. Chem. Int. Ed. Engl. 2016, 55, 10978–10999; - PubMed
-
- Velema W. A., Szymanski W., Feringa B. L., J. Am. Chem. Soc. 2014, 136, 2178–2191; - PubMed
-
- Hüll K., Morstein J., Trauner D., Chem. Rev. 2018, 118, 10710–10747. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

