Immune Persistence and Safety After SARS-CoV-2 BNT162b1 mRNA Vaccination in Chinese Adults: A Randomized, Placebo-Controlled, Double-Blind Phase 1 Trial
- PMID: 35771353
- PMCID: PMC9245386
- DOI: 10.1007/s12325-022-02206-1
Immune Persistence and Safety After SARS-CoV-2 BNT162b1 mRNA Vaccination in Chinese Adults: A Randomized, Placebo-Controlled, Double-Blind Phase 1 Trial
Abstract
Introduction: BNT162b1 is a lipid nanoparticle-formulated, nucleoside-modified mRNA SARS-CoV-2 vaccine. Here, we report safety and immune persistence data following a primary two-dose vaccination schedule administered 21 days apart.
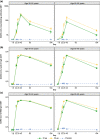
Methods: Immune persistence was determined at month 3 in 72 younger participants (aged 18-55 years) and at month 6 in 70 younger and 69 older participants (aged 65-85 years).
Results: In younger participants, neutralizing antibody (nAb) geometric mean titers (GMTs) for the 10 and 30 µg dose levels declined from 233 and 254 (21 days after dose 2) to 55 and 87 at month 3, respectively, and to 16 and 27 at month 6, respectively. In older participants, nAb GMTs declined from 80 and 160 (21 days after dose 2) to 10 and 21 at month 6. Overall, higher antibody titers were observed in younger participants, and the 30 µg dose induced higher levels of nAb, which declined more slowly by month 6. No serious adverse events were reported in the vaccine group.
Conclusion: This study showed BNT162b1 maintains a favorable safety profile in younger and older participants in the 6 months after vaccination. This study further extends our understanding of immune persistence and the safety of the BNT162b1 vaccine as a candidate vaccine in the BioNTech pipeline.
Trial registration number: NCT04523571, registered August 21, 2020.
Keywords: COVID; Immune persistence; SARS-CoV-2; mRNA vaccine.
© 2022. The Author(s), under exclusive licence to Springer Healthcare Ltd., part of Springer Nature.
Figures
References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 28 Jan 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

