Monitoring Mitochondrial Morphology and Respiration in Doxorubicin-Induced Cardiomyopathy
- PMID: 35771444
- PMCID: PMC11118012
- DOI: 10.1007/978-1-0716-2309-1_13
Monitoring Mitochondrial Morphology and Respiration in Doxorubicin-Induced Cardiomyopathy
Abstract
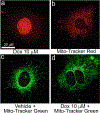
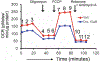
Doxorubicin (DOX)-induced cardiomyopathy constitutes dose-dependent cardiac toxicity, culminating in fatal heart failure progression. Cardiac toxicity limits effective and subsequent use of DOX in chemotherapy regimens in pediatric, adult, and recurrent cancer patients. DOX-induced profound alterations in mitochondrial morphology, dynamics, and defects in mitochondrial energy metabolism in the heart comprise key stressors in DOX-induced cardiotoxicity. Hence, the discovery of novel molecular targets and therapeutics to mitigate DOX-induced mitochondrial dysfunctions are imperative. Herein, we provided two laboratory protocols to monitor DOX-induced alterations in mitochondrial morphology and respiration in isolated primary neonatal rat cardiomyocytes. Neonatal rat cardiomyocytes are extensively used to monitor signaling mechanisms regulating cardiomyopathy in vitro. Therefore, these protocols will help researchers study the effects of novel pharmacological and genetic manipulations against DOX-induced alterations in mitochondrial morphology and energy metabolism in cardiomyocytes.
Keywords: Doxorubicin-induced cardiomyopathy; Mitochondrial morphology; Mitochondrial respiration; Oxygen consumption rates.
© 2022. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
Figures
References
-
- Bonadonna G, Monfardini S, De Lena M, Fossati-Bellani F, Beretta G (1970) Phase I and preliminary phase II evaluation of adriamycin (NSC 123127). Cancer Res 30(10): 2572–2582 - PubMed
-
- Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, Wood WC (2003) Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol 21(4):588–592 - PubMed
-
- Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA (2002) Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol 13(6):819–829 - PubMed
-
- Steinherz L, Steinherz P (1991) Delayed cardiac toxicity from anthracycline therapy. Pediatrician 18(1):49–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

