Chemogenetic inhibition of corticotropin-releasing factor neurons in the central amygdala alters binge-like ethanol consumption in male mice
- PMID: 35771510
- PMCID: PMC9671851
- DOI: 10.1037/bne0000522
Chemogenetic inhibition of corticotropin-releasing factor neurons in the central amygdala alters binge-like ethanol consumption in male mice
Abstract
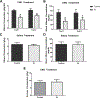
Repetitive bouts of binge drinking can lead to neuroplastic events that alter ethanol's pharmacologic effects and perpetuate excessive consumption. The corticotropin-releasing factor (CRF) system is an example of ethanol-induced neuroadaptations that drive excessive ethanol consumption. Our laboratory has previously shown that CRF antagonist, when infused into the central amygdala (CeA), reduces binge-like ethanol consumption. The present study extends this research by assessing the effects of silencing CRF-producing neurons in CeA on binge-like ethanol drinking stemming from "Drinking in the Dark" (DID) procedures. CRF-ires-Cre mice underwent surgery to infuse Gi/o-coupled Designer Receptors Exclusively Activated by Designer Drugs (DREADD) virus or a control virus into either the CeA or basolateral amygdala (BLA). Gi/o-DREADD-induced CRF-neuronal inhibition in the CeA resulted in a 33% decrease in binge-like ethanol consumption. However, no effect on ethanol consumption was seen after DREADD manipulation in the BLA. Moreover, CeA CRF-neuronal inhibition had no effect on sucrose consumption. The effects of silencing CRF neurons in the CeA on ethanol consumption are not secondary to changes in motor function or anxiety-like behaviors as assessed in the open-field test (OFT). Finally, the DREADD construct's functional ability to inhibit CRF-neuronal activity was demonstrated by reduced ethanol-induced c-Fos following DREADD activation. Together, these data suggest that the CRF neurons in the CeA play an important role in binge ethanol consumption and that inhibition of the CRF-signaling pathway remains a viable target for manipulating binge-like ethanol consumption. (PsycInfo Database Record (c) 2022 APA, all rights reserved).
Conflict of interest statement
Figures

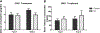

Similar articles
-
Central nucleus of the amygdala projections onto the nucleus accumbens core regulate binge-like alcohol drinking in a CRF-dependent manner.Neuropharmacology. 2022 Feb 1;203:108874. doi: 10.1016/j.neuropharm.2021.108874. Epub 2021 Nov 5. Neuropharmacology. 2022. PMID: 34748860 Free PMC article.
-
Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake.Biol Psychiatry. 2017 Jun 1;81(11):930-940. doi: 10.1016/j.biopsych.2016.02.029. Epub 2016 Mar 3. Biol Psychiatry. 2017. PMID: 27113502 Free PMC article.
-
Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice.J Neurosci. 2012 Mar 7;32(10):3405-13. doi: 10.1523/JNEUROSCI.6256-11.2012. J Neurosci. 2012. PMID: 22399763 Free PMC article.
-
Corticotrophin-releasing factor 1 activation in the central amygdale and visceral hyperalgesia.Neurogastroenterol Motil. 2015 Jan;27(1):1-6. doi: 10.1111/nmo.12495. Neurogastroenterol Motil. 2015. PMID: 25557223 Free PMC article. Review.
-
The neurobiology of binge-like ethanol drinking: evidence from rodent models.Physiol Behav. 2012 Jun 6;106(3):325-31. doi: 10.1016/j.physbeh.2011.12.026. Epub 2012 Jan 8. Physiol Behav. 2012. PMID: 22245775 Free PMC article. Review.
Cited by
-
Orexinergic lateral hypothalamus (LH) projections to medial septum (MS) modulate ethanol-induced sedation in male and female mice and binge-like ethanol drinking in male mice only.Alcohol. 2024 Mar;115:13-22. doi: 10.1016/j.alcohol.2023.09.003. Epub 2023 Sep 15. Alcohol. 2024. PMID: 37717641 Free PMC article.
-
Central amygdala neuroimmune signaling in alcohol use disorder.Addict Neurosci. 2025 Mar;14:100194. doi: 10.1016/j.addicn.2024.100194. Epub 2024 Dec 21. Addict Neurosci. 2025. PMID: 40336623 Free PMC article.
-
Inhibiting CRF Projections from the Central Amygdala to Lateral Hypothalamus and Amygdala Deletion of CRF Alters Binge-Like Ethanol Drinking in a Sex-Dependent Manner.bioRxiv [Preprint]. 2024 Apr 13:2024.04.09.588750. doi: 10.1101/2024.04.09.588750. bioRxiv. 2024. Update in: Biol Psychiatry Glob Open Sci. 2024 Oct 25;5(1):100405. doi: 10.1016/j.bpsgos.2024.100405. PMID: 38645149 Free PMC article. Updated. Preprint.
-
Mouse parasubthalamic Crh neurons drive alcohol drinking escalation and behavioral disinhibition.bioRxiv [Preprint]. 2024 Jul 10:2024.07.06.602357. doi: 10.1101/2024.07.06.602357. bioRxiv. 2024. PMID: 39026704 Free PMC article. Preprint.
References
-
- Agoglia AE, Tella J, & Herman MA (2020). Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons. Neuropharmacology, 180, 108296. doi:10.1016/j.neuropharm.2020.108296 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 AA025809/AA/NIAAA NIH HHS/United States
- P60 AA011605/AA/NIAAA NIH HHS/United States
- F31 AA027134/AA/NIAAA NIH HHS/United States
- R01 AA022048/AA/NIAAA NIH HHS/United States
- R01 AA013573/AA/NIAAA NIH HHS/United States
- R37 AA013573/AA/NIAAA NIH HHS/United States
- SC1 GM139696/GM/NIGMS NIH HHS/United States
- P50 AA011605/AA/NIAAA NIH HHS/United States
- T32 DA007244/DA/NIDA NIH HHS/United States
- F32 AA025811/AA/NIAAA NIH HHS/United States
- R25 MH129791/MH/NIMH NIH HHS/United States
- K12 GM000678/GM/NIGMS NIH HHS/United States
- F32 AA021611/AA/NIAAA NIH HHS/United States
- NH/NIH HHS/United States
- R01 AA015148/AA/NIAAA NIH HHS/United States
- K99 AA028268/AA/NIAAA NIH HHS/United States

