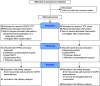
Home High-Flow Nasal Cannula Oxygen Therapy for Stable Hypercapnic COPD: A Randomized Clinical Trial
- PMID: 35771533
- PMCID: PMC9746854
- DOI: 10.1164/rccm.202201-0199OC
Home High-Flow Nasal Cannula Oxygen Therapy for Stable Hypercapnic COPD: A Randomized Clinical Trial
Abstract
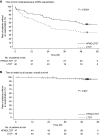
Rationale: The long-term effects of using a high-flow nasal cannula for chronic hypercapnic respiratory failure caused by chronic obstructive pulmonary disease remain unclear. Objectives: To assess whether long-term high-flow nasal cannula use reduces the number of exacerbations and improves other physiological parameters in patients with chronic hypercapnic respiratory failure caused by chronic obstructive pulmonary disease. Methods: We enrolled 104 participants (aged ⩾40 yr) with daytime hypercapnia (Global Initiative for Chronic Obstructive Lung Disease stages 2-4) receiving long-term oxygen therapy (⩾16 h/d for ⩾1 mo) and randomly assigned them to high-flow nasal cannula/long-term oxygen therapy and long-term oxygen therapy groups. The primary endpoint was the moderate or severe exacerbation rate. We compared changes from baseline in arterial blood gas values, peripheral oxygen saturation, pulmonary function, health-related quality-of-life scores, and the 6-minute-walk test. Measurements and Main Results: High-flow nasal cannula use significantly reduced the rate of moderate/severe exacerbations (unadjusted mean count 1.0 vs. 2.5, a ratio of the adjusted mean count between groups [95% confidence interval] of 2.85 [1.48-5.47]) and prolonged the duration without moderate or severe exacerbations. The median time to first moderate or severe exacerbation in the long-term oxygen therapy group was 25 (14.1-47.4) weeks; this was not reached in the high-flow nasal cannula/long-term oxygen therapy group. High-flow nasal cannula use significantly improved health-related quality of life scores, peripheral oxygen saturation, and specific pulmonary function parameters. No safety concerns were identified. Conclusions: A high-flow nasal cannula is a reasonable therapeutic option for patients with stable hypercapnic chronic obstructive pulmonary disease and a history of exacerbations. Clinical trial registered with www.umin/ac.jp (UMIN000028581) and www.clinicaltrials.gov (NCT03282019).
Keywords: chronic obstructive pulmonary disease; hypercapnia; oxygen inhalation therapy; pulmonary disease; respiratory insufficiency.
Figures

Comment in
-
High Flow Nasal Oxygen at Home to Prevent Chronic Obstructive Pulmonary Disease Exacerbations?Am J Respir Crit Care Med. 2022 Dec 1;206(11):1303-1304. doi: 10.1164/rccm.202207-1311ED. Am J Respir Crit Care Med. 2022. PMID: 35853196 Free PMC article. No abstract available.
-
High-flow Nasal Cannula Oxygen Therapy for Stable Hypercapnic COPD: Just Good Enough?Am J Respir Crit Care Med. 2022 Dec 1;206(11):1433-1434. doi: 10.1164/rccm.202207-1366LE. Am J Respir Crit Care Med. 2022. PMID: 35904804 Free PMC article. No abstract available.
-
Efficacy of High-Flow Nasal Cannula Oxygen Therapy in Reducing Future Exacerbations for Patients with Stable Hypercapnia with Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2023 Feb 15;207(4):495-496. doi: 10.1164/rccm.202210-1877LE. Am J Respir Crit Care Med. 2023. PMID: 36346704 Free PMC article. No abstract available.
References
-
- Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J . 2007;30:993–1013. - PubMed
-
- Foucher P, Baudouin N, Merati M, Pitard A, Bonniaud P, Reybet-Degat O, et al. Relative survival analysis of 252 patients with COPD receiving long-term oxygen therapy. Chest . 1998;113:1580–1587. - PubMed
-
- Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med . 2009;103:1400–1405. - PubMed
-
- Hasani A, Chapman TH, McCool D, Smith RE, Dilworth JP, Agnew JE. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis . 2008;5:81–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

