Towards network-guided neuromodulation for epilepsy
- PMID: 35771657
- PMCID: PMC9586548
- DOI: 10.1093/brain/awac234
Towards network-guided neuromodulation for epilepsy
Abstract
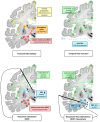
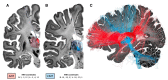
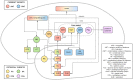
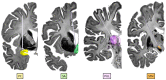
Epilepsy is well-recognized as a disorder of brain networks. There is a growing body of research to identify critical nodes within dynamic epileptic networks with the aim to target therapies that halt the onset and propagation of seizures. In parallel, intracranial neuromodulation, including deep brain stimulation and responsive neurostimulation, are well-established and expanding as therapies to reduce seizures in adults with focal-onset epilepsy; and there is emerging evidence for their efficacy in children and generalized-onset seizure disorders. The convergence of these advancing fields is driving an era of 'network-guided neuromodulation' for epilepsy. In this review, we distil the current literature on network mechanisms underlying neurostimulation for epilepsy. We discuss the modulation of key 'propagation points' in the epileptogenic network, focusing primarily on thalamic nuclei targeted in current clinical practice. These include (i) the anterior nucleus of thalamus, now a clinically approved and targeted site for open loop stimulation, and increasingly targeted for responsive neurostimulation; and (ii) the centromedian nucleus of the thalamus, a target for both deep brain stimulation and responsive neurostimulation in generalized-onset epilepsies. We discuss briefly the networks associated with other emerging neuromodulation targets, such as the pulvinar of the thalamus, piriform cortex, septal area, subthalamic nucleus, cerebellum and others. We report synergistic findings garnered from multiple modalities of investigation that have revealed structural and functional networks associated with these propagation points - including scalp and invasive EEG, and diffusion and functional MRI. We also report on intracranial recordings from implanted devices which provide us data on the dynamic networks we are aiming to modulate. Finally, we review the continuing evolution of network-guided neuromodulation for epilepsy to accelerate progress towards two translational goals: (i) to use pre-surgical network analyses to determine patient candidacy for neurostimulation for epilepsy by providing network biomarkers that predict efficacy; and (ii) to deliver precise, personalized and effective antiepileptic stimulation to prevent and arrest seizure propagation through mapping and modulation of each patients' individual epileptogenic networks.
Keywords: connectivity; deep brain stimulation; epilepsy; networks; responsive neurostimulation.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the international league against epilepsy: Position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:522–530. - PubMed
-
- Richardson MP. Large scale brain models of epilepsy: Dynamics meets connectomics. J Neurol Neurosurg Psychiatry. 2012;83:1238–1248. - PubMed
-
- Spencer SS. Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia. 2002;43:219–227. - PubMed
-
- Van Diessen E, Diederen SJHH, Braun KPJJ, Jansen FE, Stam CJ. Functional and structural brain networks in epilepsy: What have we learned? Epilepsia. 2013;54:1855–1865. - PubMed

