Specificity and Breadth of the Neutralizing Antibody Response to a Live-Attenuated Tetravalent Dengue Vaccine
- PMID: 35771658
- PMCID: PMC9704433
- DOI: 10.1093/infdis/jiac272
Specificity and Breadth of the Neutralizing Antibody Response to a Live-Attenuated Tetravalent Dengue Vaccine
Abstract
Background: An effective dengue vaccine should ideally induce broadly neutralizing antibody (nAb) responses against all 4 dengue virus (DENV) serotypes.
Methods: We characterized the specificity and breadth of the nAb response to TAK-003, a live-attenuated tetravalent dengue vaccine, in serum samples from phase 2 and 3 clinical trials.
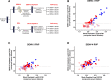
Results: Microneutralization tests using postvaccination serum showed comparable neutralization against diverse DENV-1-4 genotypes. Reporter virus particle neutralization assays after depletion of anti-DENV-2 nAbs demonstrated that the nAb response to DENV-1, -3, and -4 comprises both type-specific (TS) and cross-reactive (CR) nAbs.
Conclusions: Therefore, TAK-003 induces broad tetravalent TS and CR nAb responses.
Keywords: TAK-003; dengue vaccine; dengue virus; neutralizing antibody response; type-specific.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors are current employees of Takeda Vaccines, Inc. or were employees or contractors of Takeda Vaccines, Inc. at the time this study was conducted. HD was an employee of Takeda Vaccines, Inc at the time this work was conducted and then served as a paid consultant to Takeda Vaccines, Inc. Funding to pay the Open Access publication charges for this article was provided by Takeda Pharmaceuticals U.S.A. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Comment in
-
Immunogenicity of a Live Dengue Vaccine (TAK-003).J Infect Dis. 2022 Dec 28;227(1):163-164. doi: 10.1093/infdis/jiac424. J Infect Dis. 2022. PMID: 36285800 Free PMC article. No abstract available.
References
-
- Guzman MG, Harris E. Dengue. Lancet 2015; 385:453–65. - PubMed
-
- Gibbons RV, Kalanarooj S, Jarman RG, et al. . Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 2007; 77:910–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

