An Rtn4/Nogo-A-interacting micropeptide modulates synaptic plasticity with age
- PMID: 35771867
- PMCID: PMC9246188
- DOI: 10.1371/journal.pone.0269404
An Rtn4/Nogo-A-interacting micropeptide modulates synaptic plasticity with age
Abstract
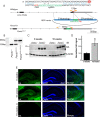
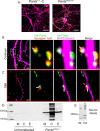
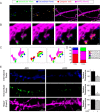
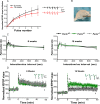
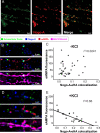
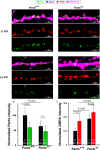
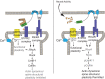
Micropeptides, encoded from small open reading frames of 300 nucleotides or less, are hidden throughout mammalian genomes, though few functional studies of micropeptides in the brain are published. Here, we describe a micropeptide known as the Plasticity-Associated Neural Transcript Short (Pants), located in the 22q11.2 region of the human genome, the microdeletion of which conveys a high risk for schizophrenia. Our data show that Pants is upregulated in early adulthood in the mossy fiber circuit of the hippocampus, where it exerts a powerful negative effect on long-term potentiation (LTP). Further, we find that Pants is secreted from neurons, where it associates with synapses but is rapidly degraded with stimulation. Pants dynamically interacts with Rtn4/Nogo-A, a well-studied regulator of adult plasticity. Pants interaction with Nogo-A augments its influence over postsynaptic AMPA receptor clustering, thus gating plasticity at adult synapses. This work shows that neural micropeptides can act as architectural modules that increase the functional diversity of the known proteome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Schneider M, Debbane M, Bassett AS, Chow EW, Fung WL, van den Bree M, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171(6):627–39. doi: 10.1176/appi.ajp.2013.13070864 - DOI - PMC - PubMed
-
- Devaraju P, Yu J, Eddins D, Mellado-Lagarde MM, Earls LR, Westmoreland JJ, et al. Haploinsufficiency of the 22q11.2 microdeletion gene Mrpl40 disrupts short-term synaptic plasticity and working memory through dysregulation of mitochondrial calcium. Mol Psychiatry. 2016. doi: 10.1038/mp.2016.75 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

