Interferon gamma release assays for diagnosis of osteoarticular tuberculosis: A systematic review and meta-analysis
- PMID: 35771875
- PMCID: PMC9246147
- DOI: 10.1371/journal.pone.0269234
Interferon gamma release assays for diagnosis of osteoarticular tuberculosis: A systematic review and meta-analysis
Abstract
Background: Although the Interferon Gamma Release Assays (IGRA) is often used to identify latent tuberculosis, it also plays a crucial role in diagnosing active extrapulmonary tuberculosis. Some studies have assessed the use of IGRA as a biomarker for osteoarticular tuberculosis (OATB), which is elevated following TB infection. Still, conclusive results about its effectiveness have not been reported.
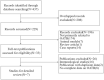
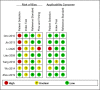
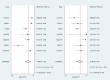
Method: We searched PubMed, Embase, and Cochran databases. We obtained literature related to the diagnosis of OATB by IGRA, and the retrieval period was from the establishment of the database to June 2021. The bivariate random effect model was used to summarize the sensitivity, specificity, and accuracy of other indicators in diagnosing OATB by IGRA, and the forest plot and receiver operating characteristic (ROC) curve were used for testing.
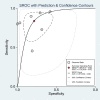
Results: We included seven studies involving 643 subjects in diagnosing OATB by IGRA. The comprehensive sensitivity and specificity were 0.84 (95% CI, 0.70-0.92) and 0.78 (95% CI, 0.66-0.87), respectively. The area under the curve (AUC) was 0.87.
Conclusion: In blood samples, the diagnostic accuracy of IGRAS is poor in patients with suspected OAT. We conclude that IGRA may not be appropriate for patients with OATB.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

