Characterization and Clinical Association of Autoantibodies Against Perilipin 1 in Patients With Acquired Generalized Lipodystrophy
- PMID: 35771980
- PMCID: PMC9797321
- DOI: 10.2337/db21-1086
Characterization and Clinical Association of Autoantibodies Against Perilipin 1 in Patients With Acquired Generalized Lipodystrophy
Abstract
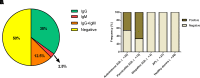
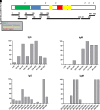
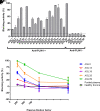
Acquired generalized lipodystrophy (AGL) is a rare condition characterized by massive loss of adipose tissue through the body, causing severe metabolic complications. Autoimmune destruction of adipocytes is strongly suspected based on the frequent association of AGL with autoimmune disorders. In 2018, autoantibodies against perilipin 1 (PLIN1) were identified in three patients with autoimmune-associated AGL. However, the pathogenic mechanism and clinical impact of anti-PLIN1 remain unsolved. The prevalence of anti-PLIN1 autoantibodies in an AGL cohort of 40 patients was 50% (20 of 40). Among positive patients, 10 had the autoimmune variety and 10 had panniculitis-associated AGL. The IgG isotype was predominant, although some IgM antibodies were detected. Epitope-mapping studies did not identify a single, major epitope. Instead, autoantibodies typically bound to several different peptides, among which the central (233-405) domain was detected in all antibody-positive patients, for both IgG and IgM autoantibodies. In-depth epitope mapping indicated that anti-PLIN1 autoantibodies predominantly recognize the αβ-hydrolase domain containing 5 (ABHD5) binding site (383-405). Autoantibodies dose-dependently blocked the binding of PLIN1 to ABHD5 and caused a dislocation of ABHD5 toward the cytosol, leading to an increase in lipolysis and lipase activities. Finally, anti-PLIN1 titers significantly correlated with the amount of fat loss, metabolic control impairment, and severity of liver injury. Our data strongly support that anti-PLIN1 autoantibodies are a diagnostic biomarker and a cause of lipodystrophy in patients with AGL.
© 2022 by the American Diabetes Association.
Figures

Comment in
-
Perilipin 1 Antibodies in Patients With Acquired Generalized Lipodystrophy.Diabetes. 2023 Jan 1;72(1):16-18. doi: 10.2337/dbi22-0022. Diabetes. 2023. PMID: 36538601 No abstract available.
References
-
- Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab 2002;87:2395. - PubMed
-
- Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003;82:129–146 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

