Oxaliplatin (3 months v 6 months) With 6 Months of Fluoropyrimidine as Adjuvant Therapy in Patients With Stage II/III Colon Cancer: KCSG CO09-07
- PMID: 35772045
- PMCID: PMC9671755
- DOI: 10.1200/JCO.21.02962
Oxaliplatin (3 months v 6 months) With 6 Months of Fluoropyrimidine as Adjuvant Therapy in Patients With Stage II/III Colon Cancer: KCSG CO09-07
Abstract
Purpose: The combination of oxaliplatin and fluoropyrimidine for 6 months is one of the standard options for adjuvant therapy for high-risk stage II and III colorectal cancers (CRCs). The optimal duration of oxaliplatin to diminish neurotoxicity without compromising efficacy needs to be clarified.
Patients and methods: This open-label, randomized, phase III, noninferiority trial randomly assigned patients with high-risk stage II and III CRC to 3 and 6 months of oxaliplatin with 6 months of fluoropyrimidine groups (3- and 6-month arms, respectively). The primary end point was disease-free survival (DFS), and the noninferiority margin was a hazard ratio (HR) of 1.25.
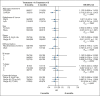
Results: In total, 1,788 patients were randomly assigned to the 6-month (n = 895) and 3-month (n = 893) arms, and 83.6% in the 6-month arm and 85.7% in the 3-month arm completed the treatment. The neuropathy rates with any grade were higher in the 6-month arm than in the 3-month arm (69.5% v 58.3%; P < .0001). The 3-year DFS rates were 83.7% and 84.7% in the 6-month and 3-month arms, respectively, with an HR of 0.953 (95% CI, 0.769 to 1.180; test for noninferiority, P = .0065) within the noninferiority margin. Among patients with stage III CRC treated by capecitabine plus oxaliplatin, the 3-year DFS of the 3-month arm was noninferior as compared with that of the 6-month arm with an HR of 0.713 (95% CI, 0.530 to 0.959; P = .0009). However, among patients with high-risk stage II and stage III CRC treated by infusional fluorouracil, leucovorin, and oxaliplatin, the noninferiority of the 3-month arm compared with the 6-month arm was not proven.
Conclusion: This study suggests that adding 3 months of oxaliplatin to 6 months of capecitabine could be considered an alternative adjuvant treatment for stage III CRC (ClinicalTrials.gov identifier: NCT01092481).
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures





References
-
- Moertel CG, Fleming TR, Macdonald JS, et al. : Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352-358, 1990 - PubMed
-
- Andre T, Boni C, Mounedji-Boudiaf L, et al. : Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343-2351, 2004 - PubMed
-
- Kuebler JP, Wieand HS, O'Connell MJ, et al. : Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol 25:2198-2204, 2007 - PubMed
-
- Haller DG, Tabernero J, Maroun J, et al. : Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 29:1465-1471, 2011 - PubMed
-
- Schmoll HJ, Van Cutsem E, Stein A, et al. : ESMO consensus guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 23:2479-2516, 2012 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

