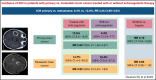
ICH in primary or metastatic brain cancer patients with or without anticoagulant treatment: a systematic review and meta-analysis
- PMID: 35772127
- PMCID: PMC9631668
- DOI: 10.1182/bloodadvances.2022008086
ICH in primary or metastatic brain cancer patients with or without anticoagulant treatment: a systematic review and meta-analysis
Abstract
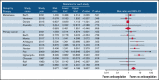
Anticoagulant treatment in patients with primary and metastatic brain cancer is a concern due to risk of intracranial hemorrhage (ICH). We performed a systematic review and meta-analysis to evaluate the risk of ICH in patients with primary or metastatic brain cancer treated with or without anticoagulants. Articles on ICH in patients with primary or metastatic brain cancer treated with or without anticoagulants published up to September 2021 were identified by searching PubMed, EMBASE, and Cochrane Library databases. The primary outcome of this analysis was ICH. Thirty studies were included. Rate of ICH was 13.0% in 1009 patients with metastatic brain cancer and 6.4% in 2353 patients with primary brain cancer (relative risk [RR], 3.26; 95% confidence interval [CI], 2.69-3.94; I2 = 92.8%). In patients with primary brain cancer, ICH occurred in 12.5% and 4.4% of patients treated with or without anticoagulants, respectively (11 studies, 659 treated and 1346 not treated patients; RR, 2.63; 95% CI, 1.48-4.67; I2 = 49.6%). In patients with metastatic brain cancer, ICH occurred in 14.7% and 15.4% (5 studies, 265 treated and 301 not treated patients; RR, 0.92; 95% CI, 0.43-1.93; I2 = 0%). ICH occurred in 8.3% of 172 treated with direct oral anticoagulants (DOACs) and in 11.7% of 278 treated with low-molecular weight heparin (LMWH) (5 studies; RR, 0.44; 95% CI, 0.25-0.79; I2 = 0%). Patients with metastatic brain cancer have a particularly high risk of ICH. Patients with primary brain cancer have an increased risk of ICH during anticoagulation. DOACs are associated with a lower risk of ICH than LMWH.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
Comment in
-
DOACs for VTE in patients with brain cancer and brain metastases: choices, choices, choices.Blood Adv. 2023 Jan 24;7(2):280-282. doi: 10.1182/bloodadvances.2022008846. Blood Adv. 2023. PMID: 36260733 Free PMC article. No abstract available.
-
DOACs in patients with brain cancers: promising but still a long way to go.Blood Adv. 2023 Jan 24;7(2):283-284. doi: 10.1182/bloodadvances.2022009192. Blood Adv. 2023. PMID: 36453644 Free PMC article. No abstract available.
References
-
- Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872. - PubMed
-
- Weinstock MJ, Uhlmann EJ, Zwicker JI. Intracranial hemorrhage in cancer patients treated with anticoagulation. Thromb Res. 2016;140(suppl 1):S60-S65. - PubMed
-
- Norden AD, Bartolomeo J, Tanaka S, et al. . Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J Neurooncol. 2012;106(1):121-125. - PubMed
-
- Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73(2):493-498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical