Incorporation of fucose into glycans independent of the GDP-fucose transporter SLC35C1 preferentially utilizes salvaged over de novo GDP-fucose
- PMID: 35772493
- PMCID: PMC9304781
- DOI: 10.1016/j.jbc.2022.102206
Incorporation of fucose into glycans independent of the GDP-fucose transporter SLC35C1 preferentially utilizes salvaged over de novo GDP-fucose
Abstract
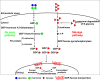
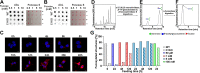
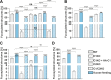
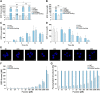
Mutations in the SLC35C1 gene encoding the Golgi GDP-fucose transporter are known to cause leukocyte adhesion deficiency II. However, improvement of fucosylation in leukocyte adhesion deficiency II patients treated with exogenous fucose suggests the existence of an SLC35C1-independent route of GDP-fucose transport, which remains a mystery. To investigate this phenomenon, we developed and characterized a human cell-based model deficient in SLC35C1 activity. The resulting cells were cultured in the presence/absence of exogenous fucose and mannose, followed by examination of fucosylation potential and nucleotide sugar levels. We found that cells displayed low but detectable levels of fucosylation in the absence of SLC35C1. Strikingly, we show that defects in fucosylation were almost completely reversed upon treatment with millimolar concentrations of fucose. Furthermore, we show that even if fucose was supplemented at nanomolar concentrations, it was still incorporated into glycans by these knockout cells. We also found that the SLC35C1-independent transport preferentially utilized GDP-fucose from the salvage pathway over the de novo biogenesis pathway as a source of this substrate. Taken together, our results imply that the Golgi systems of GDP-fucose transport discriminate between substrate pools obtained from different metabolic pathways, which suggests a functional connection between nucleotide sugar transporters and nucleotide sugar synthases.
Keywords: GDP-fucose synthesis; Golgi; LADII; N-linked glycosylation; SLC35C1; SLC35C2; cell metabolism; fucose supplementation; glycoprotein biosynthesis; membrane protein.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., et al., editors. Essentials of Glycobiology, Cold Spring Harbor Laboratory Press. Cold Spring Harbor; New York: 2015-2017. - PubMed
-
- Yurchenco P.D., Atkinson P.H. Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry. 1977;16:944–953. - PubMed
-
- Yurchenco P.D., Atkinson P.H. Fucosyl-glycoprotein and precursor polls in HeLa cells. Biochemistry. 1975;14:3107–3114. - PubMed
-
- Wiese T.J., Dunlap J.A., Yorek M.A. L-fucose is accumulated via a specific transport system in eukaryotic cells. J. Biol. Chem. 1994;269:22705–22711. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

