Importance of multiple endocrine cell types in islet organoids for type 1 diabetes treatment
- PMID: 35772687
- PMCID: PMC11554285
- DOI: 10.1016/j.trsl.2022.06.014
Importance of multiple endocrine cell types in islet organoids for type 1 diabetes treatment
Abstract
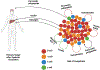
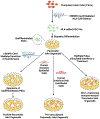
Almost 50 years ago, scientists developed the bi-hormonal abnormality hypothesis, stating that diabetes is not caused merely by the impaired insulin signaling. Instead, the presence of inappropriate level of glucagon is a prerequisite for the development of type 1 diabetes (T1D). It is widely understood that the hormones insulin and glucagon, secreted by healthy β and α cells respectively, operate in a negative feedback loop to maintain the body's blood sugar levels. Despite this fact, traditional T1D treatments rely solely on exogenous insulin injections. Furthermore, research on cell-based therapies and stem-cell derived tissues tends to focus on the replacement of β cells alone. In vivo, the pancreas is made up of 4 major endocrine cell types, that is, insulin-producing β cells, glucagon-producing α cells, somatostatin-producing δ cells, and pancreatic polypeptide-producing γ cells. These distinct cell types are involved synergistically in regulating islet functions. Therefore, it is necessary to produce a pancreatic islet organoid in vitro consisting of all these cell types that adequately replaces the function of the native islets. In this review, we describe the unique function of each pancreatic endocrine cell type and their interactions contributing to the maintenance of normoglycemia. Furthermore, we detail current sources of whole islets and techniques for their long-term expansion and culture. In addition, we highlight a vast potential of the pancreatic islet organoids for transplantation and diabetes research along with updated new approaches for successful transplantation using stem cell-derived islet organoids.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest. The authors have no conflicts of interest to declare.
Figures
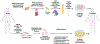
Similar articles
-
In Vivo Bioluminescence for the Detection of the Fate of Pancreatic Islet Organoids Post-transplantation.Methods Mol Biol. 2023;2592:195-206. doi: 10.1007/978-1-0716-2807-2_14. Methods Mol Biol. 2023. PMID: 36507995
-
Bi-Hormonal Endocrine Cell Presence Within the Islets of Langerhans of the Human Pancreas Throughout Life.Cells. 2025 Jan 1;14(1):34. doi: 10.3390/cells14010034. Cells. 2025. PMID: 39791735 Free PMC article.
-
Generation of Insulin-Producing Multicellular Organoids.Methods Mol Biol. 2023;2592:37-60. doi: 10.1007/978-1-0716-2807-2_3. Methods Mol Biol. 2023. PMID: 36507984
-
Recent advances in the design of implantable insulin secreting heterocellular islet organoids.Biomaterials. 2021 Feb;269:120627. doi: 10.1016/j.biomaterials.2020.120627. Epub 2020 Dec 21. Biomaterials. 2021. PMID: 33401104 Review.
-
[Insulin-secreting organoids: a first step towards the bioartificial pancreas].Med Sci (Paris). 2020 Oct;36(10):879-885. doi: 10.1051/medsci/2020129. Epub 2020 Oct 7. Med Sci (Paris). 2020. PMID: 33026330 Review. French.
Cited by
-
Pancreatic Comorbidities in Pediatric Celiac Disease: Exocrine Pancreatic Insufficiency, Pancreatitis, and Diabetes Mellitus.Diagnostics (Basel). 2025 May 14;15(10):1243. doi: 10.3390/diagnostics15101243. Diagnostics (Basel). 2025. PMID: 40428236 Free PMC article. Review.
-
Proteome Mapping of the Human Pancreatic Islet Microenvironment Reveals Endocrine-Exocrine Signaling Sphere of Influence.Mol Cell Proteomics. 2023 Aug;22(8):100592. doi: 10.1016/j.mcpro.2023.100592. Epub 2023 Jun 15. Mol Cell Proteomics. 2023. PMID: 37328065 Free PMC article.
-
Transcriptome-Powered Pluripotent Stem Cell Differentiation for Regenerative Medicine.Cells. 2023 May 22;12(10):1442. doi: 10.3390/cells12101442. Cells. 2023. PMID: 37408278 Free PMC article. Review.
-
Extracellular matrix proteins refine microenvironments for pancreatic organogenesis from induced pluripotent stem cell differentiation.Theranostics. 2025 Jan 13;15(6):2229-2249. doi: 10.7150/thno.104883. eCollection 2025. Theranostics. 2025. PMID: 39990212 Free PMC article.
-
Subcutaneous device-free islet transplantation.Front Immunol. 2023 Oct 18;14:1287182. doi: 10.3389/fimmu.2023.1287182. eCollection 2023. Front Immunol. 2023. PMID: 37965322 Free PMC article. Review.
References
-
- National Diabetes Statistics Report. Centers for Disease Control and Prevention. February 15, 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html.
-
- Diabetes. World Health Organization. 2021. https://www.who.int/news-room/fact-sheets/detail/diabetes.
-
- Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975;1:14–6. - PubMed
-
- Burcelin R, Knauf C, Cani PD. Pancreatic alpha-cell dysfunction in diabetes. Diabetes Metab 2008;34(Suppl 2):S49–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

