History, insights, and future perspectives on studies into luteal function in cattle
- PMID: 35772753
- PMCID: PMC9246667
- DOI: 10.1093/jas/skac143
History, insights, and future perspectives on studies into luteal function in cattle
Abstract
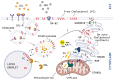
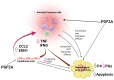
The corpus luteum (CL) forms following ovulation from the remnant of the Graafian follicle. This transient tissue produces critical hormones to maintain pregnancy, including the steroid progesterone. In cattle and other ruminants, the presence of an embryo determines if the lifespan of the CL will be prolonged to ensure successful implantation and gestation, or if the tissue will undergo destruction in the process known as luteolysis. Infertility and subfertility in dairy and beef cattle results in substantial economic loss to producers each year. In addition, this has the potential to exacerbate climate change because more animals are needed to produce high-quality protein to feed the growing world population. Successful pregnancies require coordinated regulation of uterine and ovarian function by the developing embryo. These processes are often collectively termed "maternal recognition of pregnancy." Research into the formation, function, and destruction of the bovine CL by the Northeast Multistate Project, one of the oldest continuously funded Hatch projects by the USDA, has produced a large body of evidence increasing our knowledge of the contribution of ovarian processes to fertility in ruminants. This review presents some of the seminal research into the regulation of the ruminant CL, as well as identifying mechanisms that remain to be completely validated in the bovine CL. This review also contains a broad discussion of the roles of prostaglandins, immune cells, as well as mechanisms contributing to steroidogenesis in the ruminant CL. A triadic model of luteolysis is discussed wherein the interactions among immune cells, endothelial cells, and luteal cells dictate the ability of the ruminant CL to respond to a luteolytic stimulus, along with other novel hypotheses for future research.
Keywords: bovine; corpus luteum; luteolysis; steroidogenesis.
Plain language summary
The corpus luteum (CL) forms on the ovary from the cellular remnants of the follicle following ovulation. The function of the CL is to produce progesterone that is required for successful pregnancy. In the absence of an embryo or sufficient embryonic signaling, the uterus will release a prostaglandin that kills the CL in a process called luteolysis. Therefore, the CL and the embryo share a symbiotic relationship, each requiring the other to be healthy and functional for survival. The Northeast Multistate Project, one of the oldest in the nation, has produced a large body of evidence that has enhanced our understanding of how the CL functions, its regulation, and the impact of ovarian activity on fertility of cattle. This review highlights some of the important advances made in the understanding of the ruminant CL.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Alila, H. W., and Hansel W.. . 1984a. Origin of different cell types in the bovine corpus luteum as characterized by specific monoclonal antibodies. Biol. Reprod. 31:1015–1025. - PubMed
-
- Arakane, F., Kallen C. B., Watari H., Foster J. A., Sepuri N. B. V., Pain D., Stayrook S. E., Lewis M., Gerton G. L., and Strauss J. F.. . 1998. The mechanism of action of steroidogenic acute regulatory protein (StAR): StAR acts on the outside of mitochondria to stimulate steroidogenesis. J. Biol. Chem. 273:16339–16345. doi:10.1074/jbc.273.26.16339 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

