Regulation of cellular communication network factor 1 by Ras homolog family member A in bovine steroidogenic luteal cells
- PMID: 35772754
- PMCID: PMC9246651
- DOI: 10.1093/jas/skac124
Regulation of cellular communication network factor 1 by Ras homolog family member A in bovine steroidogenic luteal cells
Abstract
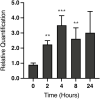
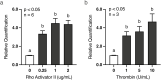
Development of the corpus luteum (CL) requires the growth of a new capillary network from preexisting vasculature, a process known as angiogenesis. Successful building of this capillary network occurs through a sequence of cellular events-differentiation, proliferation, migration, and adhesion-which are regulated by a suite of angiogenic proteins that includes cellular communication network factor 1 (CCN1). We previously reported that the expression of CCN1 was highest in luteal tissue obtained from the early-cycle, 4-d-old bovine CL (i.e., corpus hemorrhagicum) compared to the mid- and late-cycle CL. In the present study, we treated steroidogenic bovine luteal cells from early-cycle CL with luteinizing hormone (LH), but it had no effect on CCN1 expression. Direct stimulation of the canonical LH pathway with forskolin and dibutyryl-cyclic adenosine monophosphate (cAMP), however, inhibited CCN1 mRNA expression. In endothelial cells, stimulation of Ras homolog family member A (RhoA) induces CCN1 expression, whereas RhoA inactivation inhibits it. Yet, it is unknown if regulation of CCN1 in steroidogenic luteal cells works likewise. We hypothesized that a similar mechanism of CCN1 regulation exists in bovine luteal cells and that thrombin, a known RhoA activator, may be a physiologic trigger for this mechanism in the early-cycle CL. To test this hypothesis, ovaries were collected from lactating dairy cows on days 3 or 4 of the estrous cycle, and corpora lutea were dissected and dissociated. Steroidogenic luteal cells were suspended in defined Ham's F12 medium, supplemented with insulin/transferrin/selenium and gentamicin, and seeded into 6-well plates. After 24 h, spent medium was replaced with fresh Ham's F12, and the cells were cultured for 24 to 48 h. Cells were treated for 2 h with defined medium, 10% fetal bovine serum (FBS), thrombin (1, 5, 10 U/mL), or Rho Activator II (0.25, 1, 2 μg/mL). Cells were then lysed for RNA extraction, followed by cDNA generation, and quantitative polymerase chain reaction (qPCR). Thrombin (1, 5, 10 U/mL; n = 3) and Rho Activator II (0.25, 1, 2 μg/mL; n = 6) increased (P < 0.05) CCN1 mRNA expression. In summary, CCN1 in bovine steroidogenic luteal cells was induced by thrombin and appeared to be regulated in a Rho-dependent manner. Future work will elucidate the signaling partners downstream of Rho which leads to CCN1 gene expression.
Keywords: Ras homolog family member A; angiogenesis; cellular communication network factor 1; corpus luteum; luteinizing hormone.
Plain language summary
The corpus luteum (CL) is a transient ovarian endocrine gland that secretes progesterone, the hormone of pregnancy. Development of an optimally functioning CL requires the creation of a dense capillary bed through growth of new blood vessels, which is an intricate process called angiogenesis. A myriad of factors regulates angiogenesis, including the angiogenic inducer protein, cellular communication network factor 1 (CCN1). Although it is highly expressed in the early-cycle bovine CL, the mechanisms of CCN1 regulation have not been fully elucidated. In the present study, we showed that CCN1 expression in steroidogenic luteal cells from the early-cycle bovine CL was induced by Ras homolog family member A (RhoA) and by thrombin, but not by luteinizing hormone (LH). To the best of our knowledge, the involvement of thrombin and its signaling partner, RhoA, in regulating CCN1 in bovine steroidogenic luteal cells has not been previously reported. These findings will inform our future work to determine how RhoA activation by thrombin leads to increased expression of CCN1.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
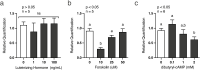
Similar articles
-
Gap junctional intercellular communication of bovine luteal cells from several stages of the estrous cycle: effects of cyclic adenosine 3',5'-monophosphate.Biol Reprod. 1996 Mar;54(3):538-45. doi: 10.1095/biolreprod54.3.538. Biol Reprod. 1996. PMID: 8835374
-
Growth hormone, but not luteinizing hormone, acts with luteal peptides on prostaglandin F2alpha and progesterone secretion by bovine corpora lutea in vitro.Prostaglandins Other Lipid Mediat. 2001 Jan;63(3):79-92. doi: 10.1016/s0090-6980(00)00099-x. Prostaglandins Other Lipid Mediat. 2001. PMID: 11204740
-
Luteal granulosa cells from natural cycles are more capable of maintaining their viability, steroidogenic activity and LH receptor expression than those of stimulated IVF cycles.Hum Reprod. 2019 Feb 1;34(2):345-355. doi: 10.1093/humrep/dey353. Hum Reprod. 2019. PMID: 30520979
-
Cell types and hormonal mechanisms associated with mid-cycle corpus luteum function.J Anim Sci. 1994 Jul;72(7):1873-83. doi: 10.2527/1994.7271873x. J Anim Sci. 1994. PMID: 7928767 Review.
-
Loss of luteal sensitivity to luteinizing hormone underlies luteolysis in cattle: A hypothesis.Reprod Biol. 2021 Dec;21(4):100570. doi: 10.1016/j.repbio.2021.100570. Epub 2021 Nov 1. Reprod Biol. 2021. PMID: 34736159 Review.
Cited by
-
A Synopsis of the NE1727 Multistate Project Collection in the Journal of Animal Science.J Anim Sci. 2022 Jul 1;100(7):skac173. doi: 10.1093/jas/skac173. J Anim Sci. 2022. PMID: 35772764 Free PMC article.
-
NE1727 Multistate Research Project: Influence of Ovary, Uterus, and Embryo on Pregnancy Success in Ruminants.J Anim Sci. 2022 Jul 1;100(7):skac178. doi: 10.1093/jas/skac178. J Anim Sci. 2022. PMID: 35772762 Free PMC article. No abstract available.
-
Mesenchymal stem cells derived exosomes: a new era in cardiac regeneration.Stem Cell Res Ther. 2025 Jan 23;16(1):16. doi: 10.1186/s13287-024-04123-2. Stem Cell Res Ther. 2025. PMID: 39849585 Free PMC article. Review.
References
-
- Bakke, L. J., Dow M., Cassar P. A., Peters M. W., Pursley J. R., and Smith G. W.. . 2002. Effect of the preovulatory gonadotropin surge on matrix metalloproteinase (MMP)-14, MMP-2, and tissue inhibitor of metalloproteinases-2 expression within bovine periovulatory follicular and luteal tissue. Biol. Reprod. 66:1627–1637. doi:10.1095/biolreprod66.6.1627. - DOI - PubMed
-
- Bakke, L. J., Li Q. L., Cassar C. A., Dow M. P. D., Pursley J. R., and Smith G. W.. . 2004. Gonadrotropin surge-induced differential upredulation of collagenase-1 (MMP-1) and collagenase-3 (MMP-3) mRNA and protein in bovine preovulatory follicles. Biol. Reprod. 71:605–612. doi:10.1095/biolreprod.104.027185. - DOI - PubMed
-
- Bassett, D. 1943. The changes in the vascular pattern of the ovary in the albino rat during the estrous cycle. Am. J. Anat. 73:251–291. doi:10.1002/aja.1000730206. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

