Arterioectatic Spinal Angiopathy of Childhood: Clinical, Imaging, Laboratory, Histologic, and Genetic Description of a Novel CNS Vascular Pathology
- PMID: 35772802
- PMCID: PMC9262071
- DOI: 10.3174/ajnr.A7551
Arterioectatic Spinal Angiopathy of Childhood: Clinical, Imaging, Laboratory, Histologic, and Genetic Description of a Novel CNS Vascular Pathology
Abstract
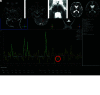
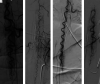
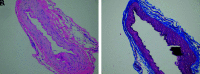
Pediatric patients with myelopathy expressing intradural spinal vascular ectasia without arteriovenous shunting were studied at four tertiary referral neuropediatric centers. Patients were identified by retrospective review of institutional records and excluded if spinal vascular pathology could be classified into a previously described category of spinal vascular malformation. Four patients meeting the study criteria were enrolled in the study. Clinical, magnetic resonance imaging, catheter-directed angiography, laboratory, histological and genetic data were analyzed to characterize the disease process and elucidate underlying pathomechanisms. Our study revealed a highly lethal, progressive multi-segmental myelopathy associated with a unique form of non-inflammatory spinal angiopathy featuring diffuse enlargement and tortuosity of spinal cord arteries, spinal cord hyperemia, and spinal cord edema (Arterioectatic Spinal Angiopathy of Childhood). The condition was shown to mimic venous congestive myelopathy associated with pediatric spinal cord arteriovenous shunts on MRI but to have distinct pathognomonic findings on catheter-directed angiography. Clinicopathological, genetic, and neuroimaging features, which are described in detail, closely overlap with those of mitochondrial disease.
© 2022 by American Journal of Neuroradiology.
Figures

Similar articles
-
A New Perspective On Arterioectatic Spinal Angiopathy with a Reversible Pattern: Cause or Consequence?Clin Neuroradiol. 2025 Mar;35(1):67-75. doi: 10.1007/s00062-024-01451-x. Epub 2024 Sep 2. Clin Neuroradiol. 2025. PMID: 39222145
-
Spinal dural arteriovenous fistula: the pathology of venous hypertensive myelopathy.Neurology. 1995 Jul;45(7):1309-13. doi: 10.1212/wnl.45.7.1309. Neurology. 1995. PMID: 7617189
-
Venous congestive myelopathy: a mimic of neoplasia.Mod Pathol. 2005 May;18(5):710-8. doi: 10.1038/modpathol.3800350. Mod Pathol. 2005. PMID: 15578073
-
Spinal cord arteriovenous shunts: from imaging to management.Eur J Radiol. 2003 Jun;46(3):221-32. doi: 10.1016/s0720-048x(03)00093-7. Eur J Radiol. 2003. PMID: 12758116 Review.
-
Vascular Diseases of the Spinal Cord: Infarction, Hemorrhage, and Venous Congestive Myelopathy.Semin Ultrasound CT MR. 2016 Oct;37(5):466-81. doi: 10.1053/j.sult.2016.05.008. Epub 2016 May 6. Semin Ultrasound CT MR. 2016. PMID: 27616317 Review.
Cited by
-
Spontaneous thrombosis of high flow pediatric arteriovenous fistulae: Case series of two patients and a comprehensive literature review.Childs Nerv Syst. 2024 May;40(5):1405-1414. doi: 10.1007/s00381-023-06241-3. Epub 2023 Dec 12. Childs Nerv Syst. 2024. PMID: 38085366 Review.
-
A New Perspective On Arterioectatic Spinal Angiopathy with a Reversible Pattern: Cause or Consequence?Clin Neuroradiol. 2025 Mar;35(1):67-75. doi: 10.1007/s00062-024-01451-x. Epub 2024 Sep 2. Clin Neuroradiol. 2025. PMID: 39222145
References
-
- Rodesch G, Hurth M, Alvarez H, et al. . Spinal cord intradural arteriovenous fistulae: anatomic, clinical, and therapeutic considerations in a series of 32 consecutive patients seen between 1981 and 2000 with emphasis on endovascular therapy. Neurosurgery 2005;57:973–83; discussion 73–83 10.1227/01.neu.0000181314.94000.cd - DOI - PubMed
