Improved Tumor Responses with Sequential Targeted α-Particles Followed by Interleukin 2 Immunocytokine Therapies in Treatment of CEA-Positive Breast and Colon Tumors in CEA Transgenic Mice
- PMID: 35772959
- PMCID: PMC9730924
- DOI: 10.2967/jnumed.122.264126
Improved Tumor Responses with Sequential Targeted α-Particles Followed by Interleukin 2 Immunocytokine Therapies in Treatment of CEA-Positive Breast and Colon Tumors in CEA Transgenic Mice
Abstract
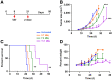
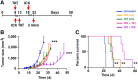
Targeted α-therapy (TAT) delivers high-linear-transfer-energy α-particles to tumors with the potential to generate tumor immune responses that may be augmented by antigen-targeted immunotherapy. Methods: This concept was evaluated in immunocompetent carcinoembryonic antigen (CEA) transgenic mice bearing CEA-positive mammary or colon tumors. Tumors were targeted with humanized anti-CEA antibody M5A labeled with 225Ac for its 10-d half-life and emission of 4 α-particles, as well as being targeted with the immunocytokine M5A-interleukin 2. Results: A dose response (3.7, 7.4, and 11.1 kBq) to TAT only, for orthotopic CEA-positive mammary tumors, was observed, with a tumor growth delay of 30 d and an increase in median survival from 20 to 36 d at the highest dose. Immunocytokine (4 times daily) monotherapy gave a tumor growth delay of 20 d that was not improved by addition of 7.4 kBq of TAT 5 d after the start of immunocytokine. However, TAT (7.4 kBq) followed by immunocytokine 10 d later led to a tumor growth delay of 38 d, with an increase in median survival to 45 d. Similar results were seen for TAT followed by immunocytokine at 5 versus 10 d. When a similar study was performed with subcutaneously implanted CEA-positive MC38 colon tumors, TAT (7.4 kBq) monotherapy gave an increase in median survival from 29 to 42 d. The addition of immunocytokine 10 d after 7.4 kBq of TAT increased median survival to 57 d. Immunophenotyping showed increased tumor-infiltrating interferon-γ-positive, CD8-positive T cells and an increased ratio of these cells to Foxp3-positive, CD4-positive regulatory T cells with sequential therapy. Immunohistochemistry confirmed there was an increase in tumor-infiltrating CD8-positive T cells in the sequential therapy group, strongly suggesting that immunocytokine augmented TAT can lead to an immune response that improves tumor therapy. Conclusion: Low-dose (7.4 kBq) TAT followed by a 4-dose immunocytokine regimen 5 or 10 d later gave superior tumor reductions and survival curves compared with either monotherapy in breast and colon cancer tumor models. Reversing the order of therapy to immunocytokine followed by TAT 5 d later was equivalent to either monotherapy in the breast cancer model.
Keywords: breast cancer; carcinoembryonic antigen; colon cancer; radionuclide therapy; targeted alpha therapy; targeted immunotherapy.
© 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Figures




References
-
- Buchegger F, Mach JP, Pelegrin A, et al. Radiolabeled chimeric anti-CEA monoclonal antibody compared with the original mouse monoclonal antibody for surgically treated colorectal carcinoma. J Nucl Med. 1995;36:420–429. - PubMed
-
- Goldenberg DM. Cancer imaging with CEA antibodies: historical and current perspectives. Int J Biol Markers. 1992;7:183–188. - PubMed
-
- Wong JY, Thomas GE, Yamauchi D, et al. Clinical evaluation of indium-111-labeled chimeric anti-CEA monoclonal antibody. J Nucl Med. 1997;38:1951–1959. - PubMed
-
- Behr TM, Sharkey RM, Juweid ME, et al. Phase I/II clinical radioimmunotherapy with an iodine-131-labeled anti-carcinoembryonic antigen murine monoclonal antibody IgG. J Nucl Med. 1997;38:858–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
