IgG N-glycome changes during the course of severe COVID-19: An observational study
- PMID: 35773089
- PMCID: PMC9234382
- DOI: 10.1016/j.ebiom.2022.104101
IgG N-glycome changes during the course of severe COVID-19: An observational study
Abstract
Background: The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causes a respiratory illness named coronavirus disease 2019 (COVID-19), which is one of the main global health problems since 2019. Glycans attached to the Fc portion of immunoglobulin G (IgG) are important modulators of IgG effector functions. Fc region binds to different receptors on the surface of various immune cells, dictating the type of immune response. Here, we performed a large longitudinal study to determine whether the severity and duration of COVID-19 are associated with altered IgG glycosylation.
Methods: Using ultra-high-performance liquid chromatography analysis of released glycans, we analysed the composition of the total IgG N-glycome longitudinally during COVID-19 from four independent cohorts. We analysed 77 severe COVID-19 cases from the HR1 cohort (74% males, median age 72, age IQR 25-80); 31 severe cases in the HR2 cohort (77% males, median age 64, age IQR 41-86), 18 mild COVID-19 cases from the UK cohort (17% males, median age 50, age IQR 26-71) and 28 mild cases from the BiH cohort (71% males, median age 60, age IQR 12-78).
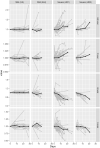
Findings: Multiple statistically significant changes in IgG glycome composition were observed during severe COVID-19. The most statistically significant changes included increased agalactosylation of IgG (meta-analysis 95% CI [0.03, 0.07], adjusted meta-analysis P= <0.0001), which regulates proinflammatory actions of IgG via complement system activation and indirectly as a lack of sialylation and decreased presence of bisecting N-acetylglucosamine on IgG (meta-analysis 95% CI [-0.11, -0.08], adjusted meta-analysis P= <0.0001), which indirectly affects antibody-dependent cell-mediated cytotoxicity. On the contrary, no statistically significant changes in IgG glycome composition were observed in patients with mild COVID-19.
Interpretation: The IgG glycome in severe COVID-19 patients is statistically significantly altered in a way that it indicates decreased immunosuppressive action of circulating immunoglobulins. The magnitude of observed changes is associated with the severity of the disease, indicating that aberrant IgG glycome composition or changes in IgG glycosylation may be an important molecular mechanism in COVID-19.
Funding: This work has been supported in part by Croatian Science Foundation under the project IP-CORONA-2020-04-2052 and Croatian National Centre of Competence in Molecular Diagnostics (The European Structural and Investment Funds grant #KK.01.2.2.03.0006), by the UKRI/MRC (Cov-0331 - MR/V027883/1) and by the National Institutes for Health Research Nottingham Biomedical Research Centre and by Ministry Of Science, Higher Education and Youth Of Canton Sarajevo, grant number 27-02-11-4375-10/21.
Keywords: Bisecting GlcNAc; COVID-19; Galactosylation; IgG glycosylation; Molecular epidemiology; SARS-CoV-2.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests G.L. is the founder and owner of Genos Ltd, a private research organization that specializes in high-throughput glycomic analysis and has several patents in this field. T.P., F.V. and I.T.A. are employees of Genos Ltd. Other authors declare no competing interests.
Figures

References
-
- Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 Accessed 8 February 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

