Targeting fibrosis, mechanisms and cilinical trials
- PMID: 35773269
- PMCID: PMC9247101
- DOI: 10.1038/s41392-022-01070-3
Targeting fibrosis, mechanisms and cilinical trials
Abstract
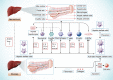
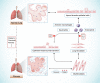
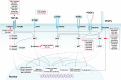
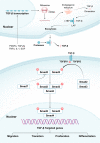
Fibrosis is characterized by the excessive extracellular matrix deposition due to dysregulated wound and connective tissue repair response. Multiple organs can develop fibrosis, including the liver, kidney, heart, and lung. Fibrosis such as liver cirrhosis, idiopathic pulmonary fibrosis, and cystic fibrosis caused substantial disease burden. Persistent abnormal activation of myofibroblasts mediated by various signals, such as transforming growth factor, platelet-derived growth factor, and fibroblast growh factor, has been recongized as a major event in the occurrence and progression of fibrosis. Although the mechanisms driving organ-specific fibrosis have not been fully elucidated, drugs targeting these identified aberrant signals have achieved potent anti-fibrotic efficacy in clinical trials. In this review, we briefly introduce the aetiology and epidemiology of several fibrosis diseases, including liver fibrosis, kidney fibrosis, cardiac fibrosis, and pulmonary fibrosis. Then, we summarise the abnormal cells (epithelial cells, endothelial cells, immune cells, and fibroblasts) and their interactions in fibrosis. In addition, we also focus on the aberrant signaling pathways and therapeutic targets that regulate myofibroblast activation, extracellular matrix cross-linking, metabolism, and inflammation in fibrosis. Finally, we discuss the anti-fibrotic drugs based on their targets and clinical trials. This review provides reference for further research on fibrosis mechanism, drug development, and clinical trials.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

