Identification of bioactive peptides from a Brazilian kefir sample, and their anti-Alzheimer potential in Drosophila melanogaster
- PMID: 35773306
- PMCID: PMC9246878
- DOI: 10.1038/s41598-022-15297-1
Identification of bioactive peptides from a Brazilian kefir sample, and their anti-Alzheimer potential in Drosophila melanogaster
Abstract
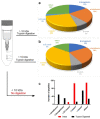
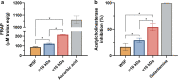
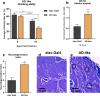
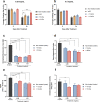
Alzheimer's disease (AD) is the most common form of dementia in the elderly, affecting cognitive, intellectual, and motor functions. Different hypotheses explain AD's mechanism, such as the amyloidogenic hypothesis. Moreover, this disease is multifactorial, and several studies have shown that gut dysbiosis and oxidative stress influence its pathogenesis. Knowing that kefir is a probiotic used in therapies to restore dysbiosis and that the bioactive peptides present in it have antioxidant properties, we explored its biotechnological potential as a source of molecules capable of modulating the amyloidogenic pathway and reducing oxidative stress, contributing to the treatment of AD. For that, we used Drosophila melanogaster model for AD (AD-like flies). Identification of bioactive peptides in the kefir sample was made by proteomic and peptidomic analyses, followed by in vitro evaluation of antioxidant and acetylcholinesterase inhibition potential. Flies were treated and their motor performance, brain morphology, and oxidative stress evaluated. Finally, we performed molecular docking between the peptides found and the main pathology-related proteins in the flies. The results showed that the fraction with the higher peptide concentration was positive for the parameters evaluated. In conclusion, these results revealed these kefir peptide-rich fractions have therapeutic potential for AD.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Brazilian kefir fraction mitigates the Alzheimer-like phenotype in Drosophila melanogaster with β-amyloid overexpression model.Sci Rep. 2024 Oct 26;14(1):25474. doi: 10.1038/s41598-024-76601-9. Sci Rep. 2024. PMID: 39461991 Free PMC article.
-
Kefir metabolites in a fly model for Alzheimer's disease.Sci Rep. 2021 May 27;11(1):11262. doi: 10.1038/s41598-021-90749-8. Sci Rep. 2021. PMID: 34045626 Free PMC article.
-
Unveiling the Brazilian kefir microbiome: discovery of a novel Lactobacillus kefiranofaciens (LkefirU) genome and in silico prospection of bioactive peptides with potential anti-Alzheimer properties.BMC Genomics. 2024 Sep 20;25(1):884. doi: 10.1186/s12864-024-10695-3. BMC Genomics. 2024. PMID: 39304820 Free PMC article.
-
The Effect of Gut Microbe Dysbiosis on the Pathogenesis of Alzheimer's Disease (AD) and Related Conditions.Curr Alzheimer Res. 2022;19(4):274-284. doi: 10.2174/1567205019666220419101205. Curr Alzheimer Res. 2022. PMID: 35440296 Review.
-
Management of oxidative stress and other pathologies in Alzheimer's disease.Arch Toxicol. 2019 Sep;93(9):2491-2513. doi: 10.1007/s00204-019-02538-y. Epub 2019 Aug 22. Arch Toxicol. 2019. PMID: 31440798 Review.
Cited by
-
Pea Peptides and Heavy Metal Neurotoxicity: Exploring Mechanisms and Mitigation Strategies in PC12 Cells.Plant Foods Hum Nutr. 2025 Mar 4;80(1):85. doi: 10.1007/s11130-025-01322-x. Plant Foods Hum Nutr. 2025. PMID: 40035902
-
The effects of Fe2O3 nanoparticles on catalytic function of human acetylcholinesterase: size and concentration role.Bioimpacts. 2024;14(5):29946. doi: 10.34172/bi.2024.29946. Epub 2024 Feb 5. Bioimpacts. 2024. PMID: 39296801 Free PMC article.
-
Clostridium butyricum improves cognitive dysfunction in ICV-STZ-induced Alzheimer's disease mice via suppressing TLR4 signaling pathway through the gut-brain axis.PLoS One. 2023 Jun 2;18(6):e0286086. doi: 10.1371/journal.pone.0286086. eCollection 2023. PLoS One. 2023. PMID: 37267300 Free PMC article.
-
Development of nutri-functional paneer whey-based kefir drink.J Food Sci Technol. 2025 Feb;62(2):254-263. doi: 10.1007/s13197-024-06023-y. Epub 2024 Jun 29. J Food Sci Technol. 2025. PMID: 39868399
-
Potential benefits of kefir and its compounds on Alzheimer's disease: A systematic review.Brain Behav Immun Integr. 2025 Apr;10:100115. doi: 10.1016/j.bbii.2025.100115. Brain Behav Immun Integr. 2025. PMID: 40376196 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

