Disassembly of α6β4-mediated hemidesmosomal adhesions promotes tumorigenesis in PTEN-negative prostate cancer by targeting plectin to focal adhesions
- PMID: 35773413
- PMCID: PMC9307480
- DOI: 10.1038/s41388-022-02389-5
Disassembly of α6β4-mediated hemidesmosomal adhesions promotes tumorigenesis in PTEN-negative prostate cancer by targeting plectin to focal adhesions
Abstract
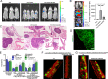
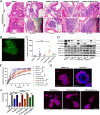
Loss of α6β4-dependent hemidesmosomal adhesions has been observed during prostate cancer progression. However, the significance and underlying mechanisms by which aberrant hemidesmosome assembly may modulate tumorigenesis remain elusive. Using an extensive CRISPR/Cas9-mediated genetic engineering approaches in different prostate cancer cell lines combined with in vivo tumorigenesis studies in mice, bone marrow-on-chip assays and bioinformatics, as well as histological analysis of prostate cancer patient cohorts, we demonstrated that simultaneous loss of PTEN and hemidesmosomal adhesions induced several tumorigenic properties including proliferation, migration, resistance to anoikis, apoptosis, and drug treatment in vitro, and increased metastatic capacity in vivo. These effects were plectin-depended and plectin was associated with actin-rich adhesions upon hemidesmosome disruption in PTEN-negative prostate cancer cells leading to activation of EGFR/PI3K/Akt- and FAK/Src-pathways. These results suggest that analysis of PTEN and hemidesmosomal proteins may have diagnostic value helping to stratify prostate cancer patients with high risk for development of aggressive disease and highlight actin-associated plectin as a potential therapeutic target specifically in PTEN/hemidesmosome dual-negative prostate cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
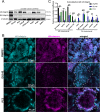
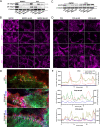
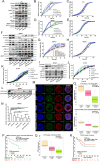
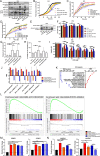


References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

