A Review on Immunological Responses to SARS-CoV-2 and Various COVID-19 Vaccine Regimens
- PMID: 35773445
- PMCID: PMC9247891
- DOI: 10.1007/s11095-022-03323-w
A Review on Immunological Responses to SARS-CoV-2 and Various COVID-19 Vaccine Regimens
Abstract
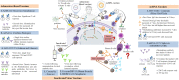
The transmission of SARS-CoV-2 has caused serious health crises globally. So far, 7 vaccines that are already being assessed in Phase IV clinical trials are, Comirnaty/ Pfizer; Spikevax/Moderna (m RNA vaccine); Vaxzevria or Covishield; Ad26.COV2.S; Ad5-nCoV (adenoviral vector-based vaccine); CoronaVac and BBIBP-CorV (inactivated virus vaccine). Besides, there are about 280 vaccines that are undergoing preclinical and clinical trials including Sputnik-V, Covaxin or BBV152, and NVX-CoV2373. These vaccines are being studied for their immunological responses and efficiency against COVID-19, and have been reported to demonstrate effective T and B cell responses. However, the long-lasting immunity of these vaccine regimens still needs to be investigated. An in-depth understanding of the vaccine efficacy and immune control mechanism is imperative for the rational purposing and implementation of the vaccines. Hence, in this review, we have comprehensively discussed the immune response induced in COVID-19 patients, as well as in the convalescent individuals to avoid reinfection. Moreover, we have also summarized the immunological responses and prophylactic efficacy of various COVID-19 vaccine regimens. In this context, this review can give insights into the development of effective vaccines against SARS-CoV-2 and its variants in the future.
Keywords: COVID-19; clinical trial; convalescent individuals; immune response; vaccine regimens.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

