Safety of Live-Attenuated Vaccines in Children Exposed to Biologic Response Modifiers in Utero
- PMID: 35773517
- PMCID: PMC11271740
- DOI: 10.1542/peds.2021-056021
Safety of Live-Attenuated Vaccines in Children Exposed to Biologic Response Modifiers in Utero
Abstract
Objectives:: To estimate the prevalence of biological response modifiers (BRM) use during pregnancy and compare clinical outcomes in infants, live-attenuated immunization coverage and adverse events of special interest (AESI) following immunization.
Methods:: We conducted a retrospective cohort study among pregnant people between 2006 – 2017 and children born from these pregnancies within the Vaccine Safety Datalink. We estimated the proportion of women who used BRM during pregnancy overall and by year of pregnancy onset. We compared clinical outcomes, live-attenuated vaccination coverage, and AESI occurring in specific risk intervals following immunization in children exposed and unexposed in utero to BRM.
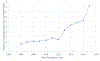
Results:: Of the 1,205,416 pregnant people, 2,243 used BRM (19/10,000), which increased from 8/10,000 in 2006 to 46/10,000 pregnant people in 2017. The most frequently dispensed or prescribed BRM were etanercept (35.9%), anakinra (23.2%), adalimumab (21.4%) and infliximab (19.9%). Except for pneumonia, clinical outcomes of interest were rare among exposed and unexposed children to BRM. Proportions of clinical outcomes were similar between both groups. A lower proportion of children exposed to BRM receive rotavirus vaccines by age 12 months compared with unexposed children (79.5% vs. 85.4%). AESI with measles-containing vaccines or rotavirus vaccines were rare and proportions of these were similar among exposed and unexposed children.
Conclusion:: In utero exposure to BRM was not associated with an increased risk of clinically significant infections or adverse events following live-attenuated vaccines. These data provide reassurance that children exposed in utero to BRM can receive live-attenuated vaccines on the same schedule as other children.
Conflict of interest statement
Dr. Klein has received research support from Sanofi Pasteur, GSK, Merck, Pfizer, and Protein Sciences (now Sanofi Pasteur) for unrelated studies. Dr. Getahun has received research grant support from NICHD, the Garfield Memorial Fund, Hologic Inc and Bayer for unrelated studies. Dr. Donahue reports grants from Janssen Global Services, LLC, outside the submitted work. Dr. Fuller has received research funding from Pfizer and Johnson & Johnson for unrelated studies.
All other co-authors have no conflicts of interest.
Figures
References
-
- Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–395. - PubMed
-
- Betteridge JD, Armbruster SP, Maydonovitch C, Veerappan GR. Inflammatory bowel disease prevalence by age, gender, race, and geographic location in the U.S. military health care population. Inflamm Bowel Dis. 2013;19(7):1421–1427. - PubMed
-
- Lerner A, Jeremias P, T M. The World Incidence and Prevalence of Autoimmune Diseases is Increasing. International Journal of Celiac Disease. 2015;3(4):151–155.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical