Are pigs overestimated as a source of zoonotic influenza viruses?
- PMID: 35773676
- PMCID: PMC9244577
- DOI: 10.1186/s40813-022-00274-x
Are pigs overestimated as a source of zoonotic influenza viruses?
Abstract
Background: Swine influenza caused by influenza A viruses (IAV) directly affects respiratory health and indirectly impairs reproduction rates in pigs causing production losses. In Europe, and elsewhere, production systems have intensified featuring fewer holdings but, in turn, increased breeding herd and litter sizes. This seems to foster swine IAV (swIAV) infections with respect to the entrenchment within and spread between holdings. Disease management of swine influenza is difficult and relies on biosecurity and vaccination measures. Recently discovered and widely proliferating forms of self-sustaining modes of swIAV infections in large swine holdings challenge these preventive concepts by generating vaccine-escape mutants in rolling circles of infection.
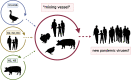
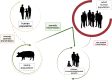
Main body: The most recent human IAV pandemic of 2009 rooted at least partly in IAV of porcine origin highlighting the zoonotic potential of swIAV. Pigs constitute a mixing vessel of IAV from different species including avian and human hosts. However, other host species such as turkey and quail but also humans themselves may also act in this way; thus, pigs are not essentially required for the generation of IAV reassortants with a multispecies origin. Since 1918, all human pandemic influenza viruses except the H2N2 virus of 1958 have been transmitted in a reverse zoonotic mode from human into swine populations. Swine populations act as long-term reservoirs of these viruses. Human-derived IAV constitute a major driver of swIAV epidemiology in pigs. Swine-to-human IAV transmissions occurred rarely and mainly sporadically as compared to avian-to-human spill-over events of avian IAV. Yet, new swIAV variants that harbor zoonotic components continue to be detected. This increases the risk that such components might eventually reassort into viruses with pandemic potential.
Conclusions: Domestic pig populations should not be globally stigmatized as the only or most important reservoir of potentially zoonotic IAV. The likely emergence from swine of the most recent human IAV pandemic in 2009, however, emphasized the principal risks of swine populations in which IAV circulate unimpededly. Implementation of regular and close-meshed IAV surveillance of domestic swine populations to follow the dynamics of swIAV evolution is clearly demanded. Improved algorithms for directly inferring zoonotic potential from whole IAV genome sequences as well as improved vaccines are still being sought.
Keywords: Mixing vessel; Reverse zoonosis; Surveillance; Swine influenza A virus; Zoonotic potential.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

