pH regulatory divergent point for the selective bio-oxidation of primary diols during resting cell catalysis
- PMID: 35773746
- PMCID: PMC9248139
- DOI: 10.1186/s13068-022-02171-5
pH regulatory divergent point for the selective bio-oxidation of primary diols during resting cell catalysis
Abstract
Background: Hydroxyl acid is an important platform chemical that covers many industrial applications due to its dual functional modules. At present, the traditional technology for hydroxyl acid production mainly adopts the petroleum route with benzene, cyclohexane, butadiene and other non-renewable resources as raw materials which violates the development law of green chemistry. Conversely, it is well-known that biotechnology and bioengineering techniques possess several advantages over chemical methods, such as moderate reaction conditions, high chemical selectivity, and environmental-friendly. However, compared with chemical engineering, there are still some major obstacles in the industrial application of biotechnology. The critical issue of the competitiveness between bioengineering and chemical engineering is products titer and volume productivity. Therefore, based on the importance of hydroxyl acids in many fields, exploring a clean, practical and environmental-friendly preparation process of the hydroxyl acids is the core purpose of this study.
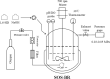
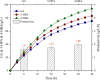
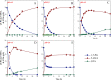
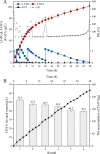
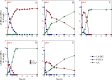
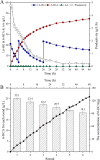
Results: To obtain high-purity hydroxyl acid, a microbiological regulation for its bioproduction by Gluconobacter oxydans was constructed. In the study, we found a critical point of chain length determine the end-products. Gluconobacter oxydans catalyzed diols with chain length ≤ 4, forming hydroxyl acids, and converting 1,5-pentylene glycol and 1,6-hexylene glycol to diacids. Based on this principle, we successfully synthesized 75.3 g/L glycolic acid, 83.2 g/L 3-hydroxypropionic acid, and 94.3 g/L 4-hydroxybutyric acid within 48 h. Furthermore, we directionally controlled the products of C5/C6 diols by adjusting pH, resulting in 102.3 g/L 5‑hydroxyvaleric acid and 48.8 g/L 6-hydroxycaproic acid instead of diacids. Combining pH regulation and cell-recycling technology in sealed-oxygen supply bioreactor, we prepared 271.4 g 5‑hydroxyvaleric acid and 129.4 g 6-hydroxycaproic acid in 6 rounds.
Conclusions: In this study, a green scheme of employing G. oxydans as biocatalyst for superior-quality hydroxyl acids (C2-C6) production is raised up. The proposed strategy commendably demonstrated a novel technology with simple pH regulation for high-value production of hydroxyl acids via green bioprocess developments.
Keywords: Hydroxyl acid; Oxidation; Sealed-oxygen supply; Whole-cell catalysis; pH regulation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- King MD, Howat IM, Candela SG, Noh MJ, Negrete A. Author correction: dynamic ice loss from the Greenland Ice Sheet driven by sustained glacier retreat. Commun Earth Environ. 2020;1:14. doi: 10.1038/s43247-020-00019-0. - DOI
-
- Gmundarson L, Herrgrd MJ, Forster J, Hauschild MZ, Fantke P. Addressing environmental sustainability of biochemicals. Nat Sustain. 2020;3:1–8. doi: 10.1038/s41893-020-0471-3. - DOI
-
- Lee SY, Kim HU, Chae TU, Cho JS, Kim JW, Shin JH, Kim DI, Ko YS, Jang WD, Jang YS. A comprehensive metabolic map for production of bio-based chemicals. Nat Catal. 2019;2:18–33. doi: 10.1038/s41929-018-0212-4. - DOI
-
- Völler J-S. Alkyne amino acid biosynthesis. Nat Catal. 2019;2:281–281. doi: 10.1038/s41929-019-0274-y. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
