Development and validation of quantitative optical index of skin blood content
- PMID: 35773754
- PMCID: PMC9243648
- DOI: 10.1117/1.JBO.27.6.065003
Development and validation of quantitative optical index of skin blood content
Abstract
Significance: We present an approach to estimate with simple instrumentation the amount of red blood cells in the skin microvasculature, designated as parameter LRBC. Variations of parameter LRBC are shown to reflect local changes in the quantity of skin red blood cells during a venous occlusion challenge.
Aim: To validate a simple algebraic model of light transport in skin using the Monte Carlo method and to develop a measure of the red blood cell content in skin microvessels using the Monte Carlo predictions; to guide the development of an instrument to measure experimentally variations of the amount of red blood cells in the skin.
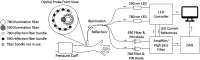
Approach: Monte Carlo simulations were carried out in a multilayer model of the skin to compute remitted light intensities as a function of distance from the illumination locus for different values of the skin blood content. The simulation results were used to compute parameter LRBC and its variations with local skin blood content. An experimental setup was developed to measure parameter LRBC in human volunteers in whom skin blood content of the forearm increased during temporary interruption of the venous outflow.
Results: In the simulations, parameter LRBC was ∼16 μm in baseline conditions, and it increased in near proportion with the blood content of the skin layers. Measuring the diffusely reflected light intensity 0.5 to 1.2 mm away from the illumination locus was optimal to detect appreciable changes of the reflected light intensity as skin blood content was altered. Parameter LRBC measured experimentally on the human forearm was 17 ± 2 μm in baseline conditions it increased at a rate of 4 ± 2 μm / min when venous outflow was temporarily interrupted.
Conclusion: Parameter LRBC derived experimentally with a two-wavelength diffuse reflectometer can be used to measure local variations of the amount of red blood cells in skin microvessels.
Keywords: Monte Carlo simulation; quantitative skin blood content; reflectance spectroscopy; tissue optics; venous occlusion.
Figures