Comparing the variability of ingredient, strength, and dose form information from electronic prescriptions with RxNorm drug product descriptions
- PMID: 35773948
- PMCID: PMC9382370
- DOI: 10.1093/jamia/ocac096
Comparing the variability of ingredient, strength, and dose form information from electronic prescriptions with RxNorm drug product descriptions
Abstract
Objective: To determine the variability of ingredient, strength, and dose form information from drug product descriptions in real-world electronic prescription (e-prescription) data.
Materials and methods: A sample of 10 399 324 e-prescriptions from 2019 to 2021 were obtained. Drug product descriptions were analyzed with a named entity extraction model and National Drug Codes (NDCs) were used to get RxNorm Concept Unique Identifiers (RxCUI) via RxNorm. The number of drug product description variants for each RxCUI was determined. Variants identified were compared to RxNorm to determine the extent of matching terminology used.
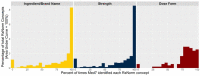
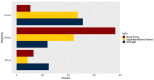
Results: A total of 353 002 unique pairs of drug product descriptions and NDCs were analyzed. The median (1st-3rd quartile) number of variants extracted for each standardized expression in RxNorm, was 3 (2-7) for ingredients, 4 (2-8) for strength, and 41 (11-122) for dosage forms. Of the pairs, 42.35% of ingredients (n = 328 032), 51.23% of strengths (n = 321 706), and 10.60% of dose forms (n = 326 653) used matching terminology, while 16.31%, 24.85%, and 13.05% contained nonmatching terminology, respectively.
Discussion: A wide variety of drug product descriptions makes it difficult to determine whether 2 drug product descriptions describe the same drug product (eg, using abbreviations to describe an active ingredient or using different units to represent a concentration). This results in patient safety risks that lead to incorrect drug products being ordered, dispensed, and used by patients. Implementation and use of standardized terminology may reduce these risks.
Conclusion: Drug product descriptions on real-world e-prescriptions exhibit large variation resulting in unnecessary ambiguity and potential patient safety risks.
Keywords: RxNorm; drug databases; electronic prescribing; medication errors; natural language processing.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Medical Informatics Association. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Rate of e-prescribing in the United States 2017–2020. https://www.statista.com/statistics/864380/share-of-us-e-prescriptions/. Accessed July 27, 2021.
-
- Lester CA, Ding Y, Li J, et al.Human versus machine editing of electronic prescription directions. J Am Pharm Assoc 2021; 61 (4): 484–91.e1. - PubMed
-
- Dhavle AA, Yang Y, Rupp MT, et al.Analysis of prescribers’ notes in electronic prescriptions in ambulatory practice. JAMA Intern Med 2016; 176 (4): 463–70. - PubMed

