Comparison of Estimated Late Toxicities between IMPT and IMRT Based on Multivariable NTCP Models for High-Risk Prostate Cancers Treated with Pelvic Nodal Radiation
- PMID: 35774485
- PMCID: PMC9238124
- DOI: 10.14338/IJPT-21-00042.1
Comparison of Estimated Late Toxicities between IMPT and IMRT Based on Multivariable NTCP Models for High-Risk Prostate Cancers Treated with Pelvic Nodal Radiation
Abstract
Purpose: To compare the late gastrointestinal (GI) and genitourinary toxicities (GU) estimated using multivariable normal tissue complication probability (NTCP) models, between pencil-beam scanning proton beam therapy (PBT) and helical tomotherapy (HT) in patients of high-risk prostate cancers requiring pelvic nodal irradiation (PNI) using moderately hypofractionated regimen.
Materials and methods: Twelve consecutive patients treated with PBT at our center were replanned with HT using the same planning goals. Six late GI and GU toxicity domains (stool frequency, rectal bleeding, fecal incontinence, dysuria, urinary incontinence, and hematuria) were estimated based on the published multivariable NTCP models. The ΔNTCP (difference in absolute NTCP between HT and PBT plans) for each of the toxicity domains was calculated. A one-sample Kolmogorov-Smirnov test was used to analyze distribution of data, and either a paired t test or a Wilcoxon matched-pair signed rank test was used to test statistical significance.
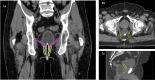
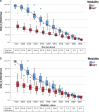
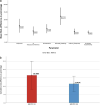
Results: Proton beam therapy and HT plans achieved adequate target coverage. Proton beam therapy plans led to significantly better sparing of bladder, rectum, and bowel bag especially in the intermediate range of 15 to 40 Gy, whereas doses to penile bulb and femoral heads were higher with PBT plans. The average ΔNTCP for grade (G)2 rectal bleeding, fecal incontinence, stool frequency, dysuria, urinary incontinence, and G1 hematuria was 12.17%, 1.67%, 2%, 5.83%, 2.42%, and 3.91%, respectively, favoring PBT plans. The average cumulative ΔNTCP for GI and GU toxicities (ΣΔNTCP) was 16.58% and 11.41%, respectively, favoring PBT. Using a model-based selection threshold of any G2 ΔNTCP >10%, 67% (8 patients) would be eligible for PBT.
Conclusion: Proton beam therapy plans led to superior sparing of organs at risk compared with HT, which translated to lower NTCP for late moderate GI and GU toxicities in patients of prostate cancer treated with PNI. For two-thirds of our patients, the difference in estimated absolute NTCP values between PBT and HT crossed the accepted threshold for minimal clinically important difference.
Keywords: NTCP; high-risk prostate cancer; late toxicities; pelvic nodal irradiation; proton therapy.
©Copyright 2022 The Author(s).
Conflict of interest statement
Conflicts of Interest: Srinivas Chilukuri, MD, has received a speaker honorarium from Astra Zeneca, maintains a leadership role in Optimus Oncology, and owns stock in Optimus Oncology. The authors have no other conflicts of interest to disclose.
Figures

References
-
- Yang DD, Nguyen PL, D'Amico AV. Elective pelvic lymph node radiation in prostate cancer revisited. J Clin Oncol . 2021;39:2845–6. - PubMed
-
- National Comprehensive Cancer Network. Prostate Version 22021 Accessed June 17 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf .
-
- European Association of Urology. EAU Guidelines Prostate Cancer Accessed June 17. 2021. https://uroweb.org/guideline/prostate-cancer/
-
- Murthy V, Maitre P, Kannan S, Panigrahi G, Krishnatry R, Bakshi G, Prakash G, Pal M, Menon S, Phurailatpam R, Mokal S, Chaurasiya D, Popat P, Sable N, Agarwal A, Rangarajan V, Joshi A, Noronha V, Prabhash K, Mahantshetty U. Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): outcomes from phase III randomized controlled trial. J Clin Oncol . 2021;39(11):1234–42. - PubMed
-
- Murthy V, Maitre P, Bhatia J, Kannan S, Krishnatry R, Prakash G, Bakshi G, Pal M, Menon S, Mahantshetty U. Late toxicity and quality of life with prostate only or whole pelvic radiation therapy in high-risk prostate cancer (POP-RT): a randomised trial. Radiother Oncol . 2020;145:71–80. - PubMed
LinkOut - more resources
Full Text Sources
