Genetics of congenital hypothyroidism: Modern concepts
- PMID: 35774517
- PMCID: PMC9218988
- DOI: 10.1002/ped4.12324
Genetics of congenital hypothyroidism: Modern concepts
Abstract
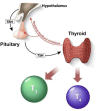
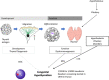
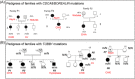
Congenital hypothyroidism (CH) is the most common neonatal endocrine disorder and one of the most common preventable causes of intellectual disability in the world. CH may be due to developmental or functional thyroid defects (primary or peripheral CH) or be hypothalamic-pituitary in origin (central CH). In most cases, primary CH is caused by a developmental malformation of the gland (thyroid dysgenesis, TD) or by a defect in thyroid hormones synthesis (dyshormonogenesis, DH). TD represents about 65% of CH and a genetic cause is currently identified in fewer than 5% of patients. The remaining 35% are cases of DH and are explained with certainty at the molecular level in more than 50% of cases. The etiology of CH is mostly unknown and may include contributions from individual and environmental factors. In recent years, the detailed phenotypic description of patients, high-throughput sequencing technologies, and the use of animal models have made it possible to discover new genes involved in the development or function of the thyroid gland. This paper reviews all the genetic causes of CH. The modes by which CH is transmitted will also be discussed, including a new oligogenic model. CH is no longer simply a dominant disease for cases of CH due to TD and recessive for cases of CH due to DH, but a far more complex disorder.
Keywords: Congenital hypothyroidism; Development; Dyshormonogenesis; Genetic; High‐throughput sequencing; Oligogenism; Thyroid dysgenesis.
© 2022 Chinese Medical Association. Pediatric Investigation published by John Wiley & Sons Australia, Ltd on behalf of Futang Research Center of Pediatric Development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Léger J. Dépistage de l'hypothyroïdie congénitale [Neonatal screening for congenital hypothyroidism]. Med Sci (Paris). 2021;37:474‐481. (in French). - PubMed
-
- Trueba SS, Augé J, Mattei G, Etchevers H, Martinovic J, Czernichow P, et al. PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis‐associated malformations. J Clin Endocrinol Metab. 2005;90:455‐462. DOI: 10.1210/jc.2004-1358 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
