Efficacy of Glutamine in Treating Severe Acute Pancreatitis: A Systematic Review and Meta-Analysis
- PMID: 35774540
- PMCID: PMC9237617
- DOI: 10.3389/fnut.2022.865102
Efficacy of Glutamine in Treating Severe Acute Pancreatitis: A Systematic Review and Meta-Analysis
Abstract
Objectives: The prognosis of severe acute pancreatitis (SAP) patients is closely related to early nutritional support. It is well-established that changes in glutamine (Gln), an important amino acid and nutritional supplement, can reflect disease severity. However, no consensus has been reached on the role of Gln nutrition therapy for SAP patients. We conducted this systematic review and meta-analysis to summarize and evaluate the advantages of Gln supplementation in SAP.
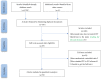
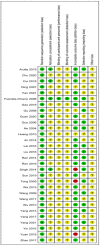
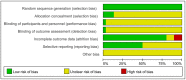
Methods: PubMed, Web of Science, the Embase, Cochrane Library, and Chinese databases (CNKI, SinoMed, Wanfang, and VIP) were systematically searched for eligible studies that included glutamine supplementation in SAP patients from inception to October 31 2021, excluding non-SAP studies. Primary outcome measures included mortality, APACHE II score, complications, and length of hospital stay. The meta-analysis was registered with PROSPERO (CRD42021288371) and was conducted using Review Manager and Stata softwares.
Results: This meta-analysis included 30 randomized controlled trials (RCTs) with a total of 1,201 patients. Six primary outcomes and six secondary outcomes were analyzed. For the primary outcomes, Gln supplementation was associated with lower mortality (OR = 0.38, 95% CI: 0.21-0.69, P = 0.001), total hospital stay (MD = -3.41, 95% CI: -4.93 to -1.88, P < 0.0001) and complications (OR = 0.45, 95% CI: 0.31-0.66, P < 0.0001) compared with conventional nutrition. Further subgroup analysis found that parenteral glutamine was more effective in reducing mortality. In terms of secondary outcomes, Gln supplementation helped restore liver, kidney and immune function, with significantly increased serum albumin (SMD = 1.02, 95% CI: 0.74-1.31, P < 0.00001) and IgG levels (MD = 1.24, 95% CI: 0.82-1.67, P < 0.00001), and decreased serum creatinine (Scr) (MD = -12.60, 95% CI: -21.97 to -3.24, P = 0.008), and inflammatory indicators such as C-reaction protein (CRP) (SMD = -1.67, 95% CI: -2.43 to -0.90, P < 0.0001).
Conclusion: Although Gln supplementation is not routinely recommended, it is beneficial for SAP patients. Indeed, glutamine nutrition has little effect on some indicator outcomes but contributes to improving the prognosis of this patient population.Systematic Review Registration: PROSPERO (york.ac.uk). Unique Identifier: CRD42021288371.
Keywords: glutamine; meta-analysis; prognosis; severe acute pancreatitis; treatment.
Copyright © 2022 Dong, Zhao, Li, Chen, Jiang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


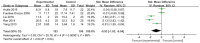

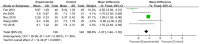




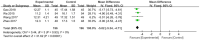
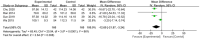



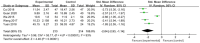

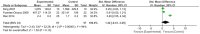
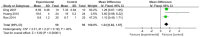
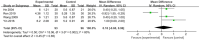

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

