Spironolactone Versus Oral Contraceptive Pills in the Treatment of Adolescent Polycystic Ovarian Syndrome: A Systematic Review
- PMID: 35774693
- PMCID: PMC9236646
- DOI: 10.7759/cureus.25340
Spironolactone Versus Oral Contraceptive Pills in the Treatment of Adolescent Polycystic Ovarian Syndrome: A Systematic Review
Abstract
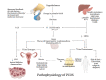
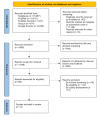
Polycystic ovarian syndrome (PCOS) is a multi-system endocrinopathy that affects women of reproductive age. Due to features that coincide with puberty, it frequently remains undiagnosed in adolescent females. The lack of evidence on management alternatives has resulted in significant variation in practice. This systematic review evaluated the therapeutic advantages and adverse effects of a regularly used therapy option, combined oral contraceptive pills (COC/OCP) with spironolactone (SP), a newer alternative that may be used alone or in conjunction with other drugs to treat adolescent PCOS. A literature search was conducted using PubMed, PubMed Central, Scopus, and Google Scholar. It was restricted to studies published in English between 2021 and 2011 that discussed the management of adolescent PCOS with COC, SP, or both. The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines. Two reviewers independently examined the content of the included studies using appropriate quality assessment tools. Four meta-analyses, four randomized controlled trials (RCTs), and one traditional review were found to be eligible. After extensive analysis, we concluded that SP, alone or in combination, is far safer than COC. However, COC treats more PCOS-associated symptoms than SP, including acne and menstrual irregularities, while also providing contraceptive benefits. However, SP monotherapy is cardioprotective and therapeutic when combined with other drugs. Long-term COC use has been linked to an increased risk of venous thromboembolism, hypertension, dyslipidemia, low-density lipoprotein (LDL) elevation, dysglycemia, and cancer in women.
Keywords: adolescent; adolescent pcos; coc; combined oral contraceptive pills; ocp; oral contraceptive pills; pcos; polycystic ovarian syndrome; spiomet; spironolactone.
Copyright © 2022, Rajashekar et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Escobar-Morreale HF. Nat Rev Endocrinol. 2018;14:270–284. - PubMed
-
- Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. Azziz R, Carmina E, Dewailly D, et al. J Clin Endocrinol Metab. 2006;91:4237–4245. - PubMed
-
- Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. Ndefo UA, Eaton A, Green MR. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3737989/ P T. 2013;38:336–355. - PMC - PubMed
-
- The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. Hum Reprod. 2016;31:2841–2855. - PubMed
-
- Polycystic ovary syndrome: a common endocrine disorder and risk factor for vascular disease. McGowan MP. Curr Treat Options Cardiovasc Med. 2011;13:289–301. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
