A Recombinant MVA-Based RSV Vaccine Induces T-Cell and Antibody Responses That Cooperate in the Protection Against RSV Infection
- PMID: 35774800
- PMCID: PMC9238321
- DOI: 10.3389/fimmu.2022.841471
A Recombinant MVA-Based RSV Vaccine Induces T-Cell and Antibody Responses That Cooperate in the Protection Against RSV Infection
Abstract
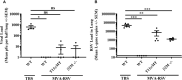
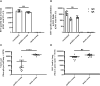
Respiratory syncytial virus (RSV) causes a respiratory disease with a potentially fatal outcome especially in infants and elderly individuals. Several vaccines failed in pivotal clinical trials, and to date, no vaccine against RSV has been licensed. We have developed an RSV vaccine based on the recombinant Modified Vaccinia Virus Ankara-BN® (MVA-RSV), containing five RSV-specific antigens that induced antibody and T-cell responses, which is currently tested in clinical trials. Here, the immunological mechanisms of protection were evaluated to determine viral loads in lungs upon vaccination of mice with MVA-RSV followed by intranasal RSV challenge. Depletion of CD4 or CD8 T cells, serum transfer, and the use of genetically engineered mice lacking the ability to generate either RSV-specific antibodies (T11µMT), the IgA isotype (IgA knockout), or CD8 T cells (β2M knockout) revealed that complete protection from RSV challenge is dependent on CD4 and CD8 T cells as well as antibodies, including IgA. Thus, MVA-RSV vaccination optimally protects against RSV infection by employing multiple arms of the adaptive immune system.
Keywords: MVA; RSV; mode of action; protection; vaccines.
Copyright © 2022 Endt, Wollmann, Haug, Bernig, Feigl, Heiseke, Kalla, Hochrein, Suter, Chaplin and Volkmann.
Conflict of interest statement
KE, JH, CB, MF, MK, HH, AV, and PC are employees of Bavarian Nordic. YW and AH were employed by Bavarian Nordic at the time of conducting this study. MS is employed as a consultant at Bavarian Nordic. The authors declare that the study was funded by Bavarian Nordic. The funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
Figures


References
-
- Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, et al. . Impaired Antibody-Mediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults With Respiratory Syncytial Virus. Am J Respir Crit Care Med (2015) 191(9):1040–9. doi: 10.1164/rccm.201412-2256OC - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

