Functional Characterization of UDP-Glycosyltransferases Involved in Anti-viral Lignan Glycosides Biosynthesis in Isatis indigotica
- PMID: 35774804
- PMCID: PMC9237620
- DOI: 10.3389/fpls.2022.921815
Functional Characterization of UDP-Glycosyltransferases Involved in Anti-viral Lignan Glycosides Biosynthesis in Isatis indigotica
Abstract
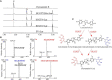
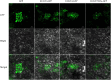
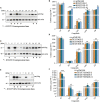
Isatis indigotica is a popular herbal medicine with its noticeable antiviral properties, which are primarily due to its lignan glycosides such as lariciresinol-4-O-β-D-glucoside and lariciresinol-4,4'-bis-O-β-D-glucosides (also called clemastanin B). UDP-glucose-dependent glycosyltransferases are the key enzymes involved in the biosynthesis of these antiviral metabolites. In this study, we systematically characterized the UGT72 family gene IiUGT1 and two UGT71B family genes, IiUGT4 and IiUGT71B5a, with similar enzymatic functions. Kinetic analysis showed that IiUGT4 was more efficient than IiUGT1 or IiUGT71B5a for the glycosylation of lariciresinol. Further knock-down and overexpression of these IiUGTs in I. indigotica's hairy roots indicates that they play different roles in planta: IiUGT71B5a primarily participates in the biosynthesis of coniferin not pinoresinol diglucoside, and IiUGT1 primarily participates in the biosynthesis of pinoresinol diglucoside, while IiUGT4 is responsible for the glycosylation of lariciresinol and plays a dominant role in the biosynthesis of lariciresinol glycosides in I. indigotica. Analysis of the molecular docking and site-mutagenesis of IiUGT4 have found that key residues for its catalytic activity are H373, W376, E397, and that F151 could be associated with substrate preference. This study elucidates the biosynthetic route of anti-viral lignan glycosides in I. indigotica, and provides the foundation for the production of anti-viral lignan glycosides via synthetic biology under the heterologous model.
Keywords: Isatis indigotica; clemastanin B; diversification; glycosyltransferases; lignan biosynthesis; transgenic hairy roots.
Copyright © 2022 Tan, Yang, Jiang, Wang, Liu, Zhao, Jin, Wang, Chen, Kang, Guo, Cui, Tang and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Atsushi O., Tatsuya K., Eiichiro O., Hyun J., Honoo S., Bun-ichi S., et al. (2014). Glucosyltransferase activity of Arabidopsis UGT71C1 towards pinoresinol and lariciresinol. Plant Biotechnol. 31 561–566. 10.5511/plantbiotechnology.14.0910a - DOI
-
- Beijing N. P. C. (2020). Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science and Technology Press.
LinkOut - more resources
Full Text Sources
Miscellaneous

