Hydroxyproline- O-Galactosyltransferases Synthesizing Type II Arabinogalactans Are Essential for Male Gametophytic Development in Arabidopsis
- PMID: 35774810
- PMCID: PMC9237623
- DOI: 10.3389/fpls.2022.935413
Hydroxyproline- O-Galactosyltransferases Synthesizing Type II Arabinogalactans Are Essential for Male Gametophytic Development in Arabidopsis
Abstract
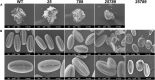
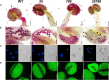
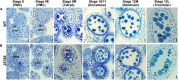
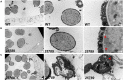
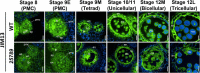
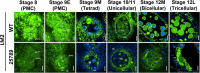
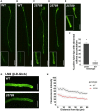
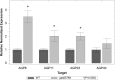
In flowering plants, male reproductive function is determined by successful development and performance of stamens, pollen grains, and pollen tubes. Despite the crucial role of highly glycosylated arabinogalactan-proteins (AGPs) in male gamete formation, pollen grain, and pollen tube cell walls, the underlying mechanisms defining these functions of AGPs have remained elusive. Eight partially redundant Hyp-galactosyltransferases (named GALT2-GALT9) genes/enzymes are known to initiate Hyp-O-galactosylation for Hyp-arabinogalactan (AG) production in Arabidopsis thaliana. To assess the contributions of these Hyp-AGs to male reproductive function, we used a galt2galt5galt7galt8galt9 quintuple Hyp-GALT mutant for this study. Both anther size and pollen viability were compromised in the quintuple mutants. Defects in male gametogenesis were observed in later stages of maturing microspores after meiosis, accompanied by membrane blebbing and numerous lytic vacuoles. Cytological and ultramicroscopic observations revealed that pollen exine reticulate architecture and intine layer development were affected such that non-viable collapsed mature pollen grains were produced, which were devoid of cell content and nuclei, with virtually no intine. AGP immunolabeling demonstrated alterations in cell wall architecture of the anther, pollen grains, and pollen tube. Specifically, the LM2 monoclonal antibody (which recognized β-GlcA epitopes on AGPs) showed a weak signal for the endothecium, microspores, and pollen tube apex. Pollen tube tips also displayed excessive callose deposition. Interestingly, expression patterns of pollen-specific AGPs, namely AGP6, AGP11, AGP23, and AGP40, were determined to be higher in the quintuple mutants. Taken together, our data illustrate the importance of type-II AGs in male reproductive function for successful fertilization.
Keywords: arabinogalactan-proteins; exine; hydroxyproline-galactosyltransferases; intine; microgametogenesis; pollen grains; pollen tube.
Copyright © 2022 Kaur, Moreira, Coimbra and Showalter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Basu D., Tian L., Wang W., Bobbs S., Herock H., Travers A., et al. (2015a). A small multigene hydroxyproline-O-galactosyltransferase family functions in arabinogalactan-protein glycosylation, growth and development in Arabidopsis. BMC Plant Biol. 15:295. 10.1186/s12870-015-0670-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

