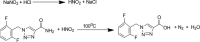Development and Validation of Chromatographic and Spectrophotometric Methods for the Quantitation of Rufinamide in Pharmaceutical Preparations
- PMID: 35775239
- PMCID: PMC9254087
- DOI: 10.4274/tjps.galenos.2021.37043
Development and Validation of Chromatographic and Spectrophotometric Methods for the Quantitation of Rufinamide in Pharmaceutical Preparations
Abstract
Objectives: Two optimized and validated high performance liquid chromatography (HPLC) and spectrophotometric methods are proposed. The developed methods were quantified with high sensitivity, accuracy, and precision at low concentrations to determine rufinamide (RUF) in active pharmaceutical ingredients (API) and pharmaceutical preparations.
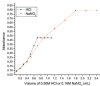
Materials and methods: HPLC method was developed using a base deactivated silica Hypersil C18 column and a combination of methanol: acetonitrile: water (15: 10: 75, v/v/v) as the mobile phase and detected at 210 nm. A reaction of RUF with sodium nitrite and hydrochloric acid occurred, absorbed maximally at 385 nm was extended to develop a ultraviolet (UV)-visible spectrophotometric method to determine RUF in API and pharmaceutical preparations.
Results: Different analytical validation parameters, including specificity, linearity, accuracy, precision, the limit of detection, quantification, ruggedness, and robustness, were determined as per International Conference on Harmonization guidelines. The linearity range of RUF was 0.15-3.5 and 10-100 μg/mL for HPLC and spectrophotometric methods, respectively.
Conclusion: The proposed investigations were valuable for drug monitoring and regular analysis of RUF in quality control and research laboratories. Moreover, the accuracy and precision obtained with the UV-visible spectrophotometer implied that it could be a cheap, easy, and alternative method, while HPLC could be sensitive to determine RUF at low concentration levels.
Keywords: HPLC; Rufinamide; UV-visible spectrophotometry; quality control laboratories; validation.
©Turk J Pharm Sci, Published by Galenos Publishing House.
Conflict of interest statement
Conflict of Interest: No conflict of interest was declared by the authors.
Figures
Similar articles
-
Validation of HPLC and UV spectrophotometric methods for the determination of meropenem in pharmaceutical dosage form.J Pharm Biomed Anal. 2003 Dec 4;33(5):947-54. doi: 10.1016/s0731-7085(03)00366-2. J Pharm Biomed Anal. 2003. PMID: 14656585
-
Development and validation of RP-HPLC and ultraviolet spectrophotometric methods of analysis for the quantitative estimation of antiretroviral drugs in pharmaceutical dosage forms.J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Jan 18;830(2):349-54. doi: 10.1016/j.jchromb.2005.11.014. Epub 2005 Dec 5. J Chromatogr B Analyt Technol Biomed Life Sci. 2006. PMID: 16330261
-
Development and validation of an high-performance liquid chromatographic, and a ultraviolet spectrophotometric method for determination of Ambroxol hydrochloride in pharmaceutical preparations.J Adv Pharm Technol Res. 2013 Jan;4(1):65-8. doi: 10.4103/2231-4040.107503. J Adv Pharm Technol Res. 2013. PMID: 23662284 Free PMC article.
-
Development and validation of column high-performance liquid chromatographic and ultraviolet spectrophotometric methods for citalopram in tablets.J AOAC Int. 2008 Jan-Feb;91(1):52-8. J AOAC Int. 2008. PMID: 18376585
-
Comparison of UV spectrophotometry and high performance liquid chromatography methods for the determination of repaglinide in tablets.Pharm Methods. 2012 Jul;3(2):68-72. doi: 10.4103/2229-4708.103875. Pharm Methods. 2012. PMID: 23781481 Free PMC article.
Cited by
-
A novel fluorescence-based method for the determination of Zuranolone using Tinopal CBS-X in the pharmaceutical formulation and spiked human plasma.Sci Rep. 2025 Jul 9;15(1):24778. doi: 10.1038/s41598-025-07726-8. Sci Rep. 2025. PMID: 40634335 Free PMC article.
References
-
- Kim SH, Kang HC, Lee JS, Kim HD. Rufinamide efficacy and safety in children aged 1-4 years with Lennox-Gastaut syndrome. Brain Dev. 2018;40:897–903. - PubMed
-
- Saydam M, Takka S. Bioavailability file: rufinamide. FABAD J Pharm Sci. 2019;44:231–242.
-
- Cheng-Hakimian A, Anderson GD, Miller JW. Rufinamide: pharmacology, clinical trials, and role in clinical practice. Int J Clin Pract. 2006;60:1497–1501. - PubMed
-
- Kluger G, Glauser T, Krauss G, Seeruthun R, Perdomo C, Arroyo S. Adjunctive rufinamide in Lennox-Gastaut syndrome: a long-term, open-label extension study. Acta Neurol Scand. 2010;122:202–208. - PubMed
LinkOut - more resources
Full Text Sources