CYP11A1‑derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas
- PMID: 35775377
- PMCID: PMC9262157
- DOI: 10.3892/ijo.2022.5386
CYP11A1‑derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas
Abstract
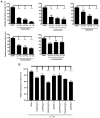
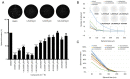
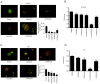
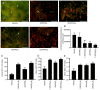
Hydroxyderivatives of vitamin D3, including classical 1,25(OH)2D3 and novel CYP11A1‑derived hydroxyderivatives, exert their biological activity by acting as agonists on the vitamin D receptor (VDR) and inverse agonists on retinoid‑related orphan receptors (ROR)α and γ. The anticancer activities of CYP11A1‑derived hydroxyderivatives were tested using cell biology, tumor biology and molecular biology methods in human A431 and SCC13 squamous (SCC)‑ and murine ASZ001 basal (BCC)‑cell carcinomas, in comparison with classical 1,25(OH)2D3. Vitamin D3‑hydroxyderivatives with or without a C1α(OH) inhibited cell proliferation in a dose‑dependent manner. While all the compounds tested had similar effects on spheroid formation by A431 and SCC13 cells, those with a C1α(OH) group were more potent in inhibiting colony and spheroid formation in the BCC line. Potent anti‑tumorigenic activity against the BCC line was exerted by 1,25(OH)2D3, 1,20(OH)2D3, 1,20,23(OH)3D3, 1,20,24(OH)3D3, 1,20,25(OH)3D3 and 1,20,26(OH)3D3, with smaller effects seen for 25(OH)D3, 20(OH)D3 and 20,23(OH)2D3. 1,25(OH)2D3, 1,20(OH)2D3 and 20(OH)D3 inhibited the expression of GLI1 and β‑catenin in ASZ001 cells. In A431 cells, these compounds also decreased the expression of GLI1 and stimulated involucrin expression. VDR, RORγ, RORα and CYP27B1 were detected in A431, SCC13 and ASZ001 lines, however, with different expression patterns. Immunohistochemistry performed on human skin with SCC and BCC showed nuclear expression of all three of these receptors, as well as megalin (transmembrane receptor for vitamin D‑binding protein), the level of which was dependent on the type of cancer and antigen tested in comparison with normal epidermis. Classical and CYP11A1‑derived vitamin D3‑derivatives exhibited anticancer‑activities on skin cancer cell lines and inhibited GLI1 and β‑catenin signaling in a manner that was dependent on the position of hydroxyl groups. The observed expression of VDR, RORγ, RORα and megalin in human SCC and BCC suggested that they might provide targets for endogenously produced or exogenously applied vitamin D hydroxyderivatives and provide excellent candidates for anti‑cancer therapy.
Keywords: basal cell carcinoma; keratinocytes; retinoid‑related orphan receptor γ; retinoid‑related orphan receptor α; squamous cell carcinoma; vitamin D; vitamin D receptor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Wondrak GT. Let the sun shine in: Mechanisms and potential for therapeutics in skin photodamage. Curr Opin Investig Drugs. 2007;8:390–400. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
