Bone marrow CD3+ CD56+ regulatory T lymphocytes (TR3 -56 cells) are inversely associated with activation and expansion of bone marrow cytotoxic T cells in IPSS-R very-low/low risk MDS patients
- PMID: 35775392
- PMCID: PMC9543123
- DOI: 10.1111/ejh.13822
Bone marrow CD3+ CD56+ regulatory T lymphocytes (TR3 -56 cells) are inversely associated with activation and expansion of bone marrow cytotoxic T cells in IPSS-R very-low/low risk MDS patients
Abstract
Background: Emergence of dysplastic haematopoietic precursor/s, cytopenia and variable leukaemia risk characterise myelodysplastic syndromes (MDS). Impaired immune-regulation, preferentially affecting cytotoxic T cells (CTL), has been largely observed in MDS. Recently, we described the TR3-56 T cell subset, characterised by the co-expression of CD3 and CD56, as a novel immune-regulatory population, able to modulate cytotoxic functions. Here, we address the involvement of TR3-56 cells in MDS pathogenesis/progression.
Objectives: To analyse the relationship between TR3-56 and CTL activation/expansion in bone marrow (BM) of very-low/low-risk MDS subjects.
Methods: Peripheral blood and BM specimens, obtained at disease onset in a cohort of 58 subjects, were analysed by immune-fluorescence and flow cytometry, to preserve the complexity of the biological sample.
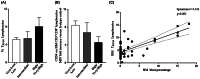
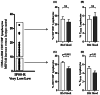
Results: We observed that a trend-increase of BM TR3-56 in high/very-high MDS stage, as compared with very-low/low group, associates with a decreased activation of BM resident CTL; significant correlation of TR3-56 with BM blasts has been also revealed. In addition, in very-low/low-risk subjects the TR3-56 amount in BM inversely correlates with the presence of activated BM CTL showing a skewed Vβ T-cell repertoire.
Conclusions: These data add TR3-56 to the immune-regulatory network involved in MDS pathogenesis/progression. Better knowledge of the immune-mediated processes associated with the disease might improve MDS clinical management.
Keywords: T lymphocytes regulatory; bone marrow; cytotoxic T-lymphocytes; myelodysplastic syndrome.
© 2022 The Authors. European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
The author declare no conflict of interest.
Figures

Comment in
-
Persistent decreased bone marrow CD3+ CD56+ T lymphocytes are inversely associated with mature granulocytes in myelodysplastic syndromes.Eur J Haematol. 2023 May;110(5):575-577. doi: 10.1111/ejh.13932. Epub 2023 Feb 5. Eur J Haematol. 2023. PMID: 36660773 No abstract available.
References
MeSH terms
Grants and funding
- FISM-Fondazione Italiana Sclerosi Multipla - 2017/R/23 and financed or co-financed with the '5 per mille' public funding to Dr G. Ruggiero
- Programma Operativo Nazionale "Ricerca e Innovazione" 2014-2020 (PON "R&I" 2014-2020), for the project "Prodotti innovativi ad alto contenuto biotecnologico- INBIOMED" (Ministero Italiano dell'Università e della Ricerca, code ARS01_01081) to Dr G. Terrazzano
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

