The effect of curcumin on hepatic fat content in individuals with obesity
- PMID: 35775631
- PMCID: PMC9804166
- DOI: 10.1111/dom.14804
The effect of curcumin on hepatic fat content in individuals with obesity
Abstract
Aim: To evaluate the effect of curcumin treatment on hepatic fat content in obese individuals.
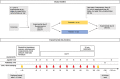
Materials and methods: In a double-blind, parallel-group trial, 37 obese, non-diabetic individuals were randomized to placebo or curcumin treatment for 6 weeks. Curcumin was dosed as lecithin-formulated tablet; 200 mg twice daily. The primary endpoint was hepatic fat content as assessed by magnetic resonance spectroscopy (MRS). Other endpoints included anthropometric measurements, hepatic biomarkers including FibroScan measurements, metabolic variables, inflammation markers, appetite measures and ad libitum food intake.
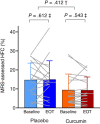
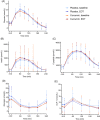
Results: Baseline characteristics (mean ± SD) were age 46 ± 14 years, hepatic fat content 12.2% ± 8.8% points, body mass index 38.8 ± 6.1 kg/m2 and waist circumference 125.8 ± 12.3 cm. After 6 weeks of treatment with curcumin, hepatic fat content was changed by -0.86% points (95% CI -3.65; 1.94) compared with 0.71% points (95% CI - 2.08; 3.51) with placebo, thus resulting in a non-significant estimated treatment difference of -1.57% points (95% CI -5.36; 2.22, P = .412). Compared with placebo, curcumin treatment caused small reductions in fasting plasma glucose (estimated treatment difference [ETD] - 0.24 mmol/L [95% CI -0.45; -0.03]), triglycerides (ETD [percentage change] -20.22% [95% CI -33.21; -6.03]) and gamma glutamyltransferase (ETD [percentage change] -15.70% [95% CI -23.32; -7.32]), but except for gamma glutamyltransferase, none of these differences remained statistically significant after adjusting for multiple testing. Treatment was well tolerated.
Conclusions: Compared with placebo, curcumin treatment for 6 weeks had no significant effect on MRS-assessed hepatic fat content in obese individuals with primarily mild steatosis. Curcumin was well tolerated.
Keywords: clinical trial; fatty liver disease; insulin resistance; phenol; randomized trial.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that there are no conflicts of interests associated with this manuscript.
Figures
References
-
- Younossi ZM. Non‐alcoholic fatty liver disease ‐ A global public health perspective. J Hepatol. 2019;70(3):531‐544. - PubMed
-
- Aller R, Fernández‐Rodríguez C, Lo Iacono O, et al. Consensus document. Management of non‐alcoholic fatty liver disease (NAFLD). Clinical practice guideline. Gastroenterol Hepatol. 2018;41(5):328‐349. - PubMed
-
- Polyzos SA, Kang ES, Boutari C, Rhee EJ, Mantzoros CS. Current and emerging pharmacological options for the treatment of nonalcoholic steatohepatitis. Metabolism. 2020;111S:154203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

